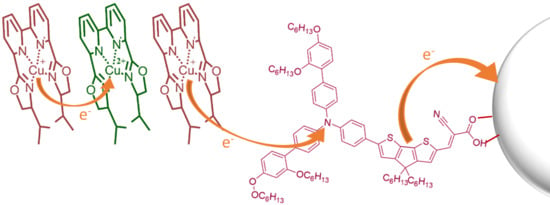
Copper Complexes with Tetradentate Ligands for Enhanced Charge Transport in Dye-Sensitized Solar Cells
Abstract
:1. Introduction
2. Results and Discussion
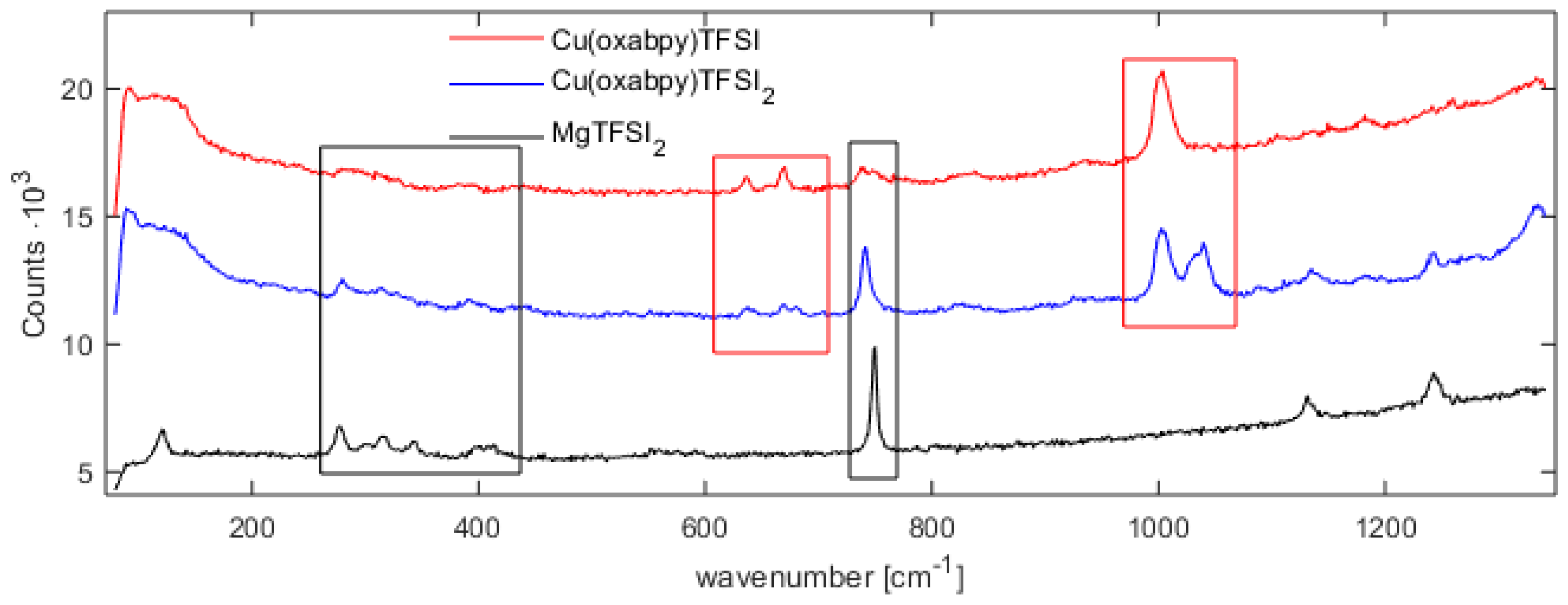
2.1. Characterization of Cu(oxabpy) Complexes
2.2. Density Functional Theory Calculations
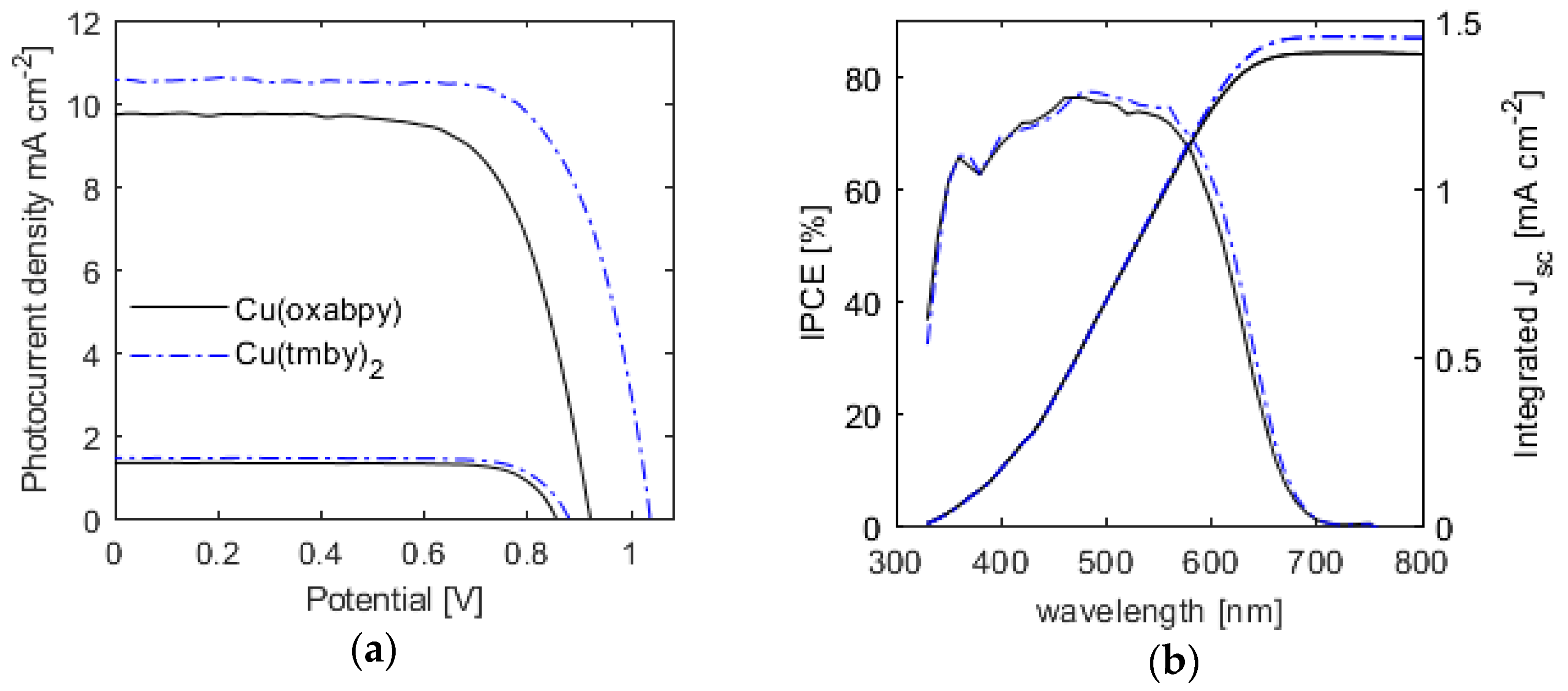
2.3. Photovoltaic Performance in Dye-Sensitized Solar Cells
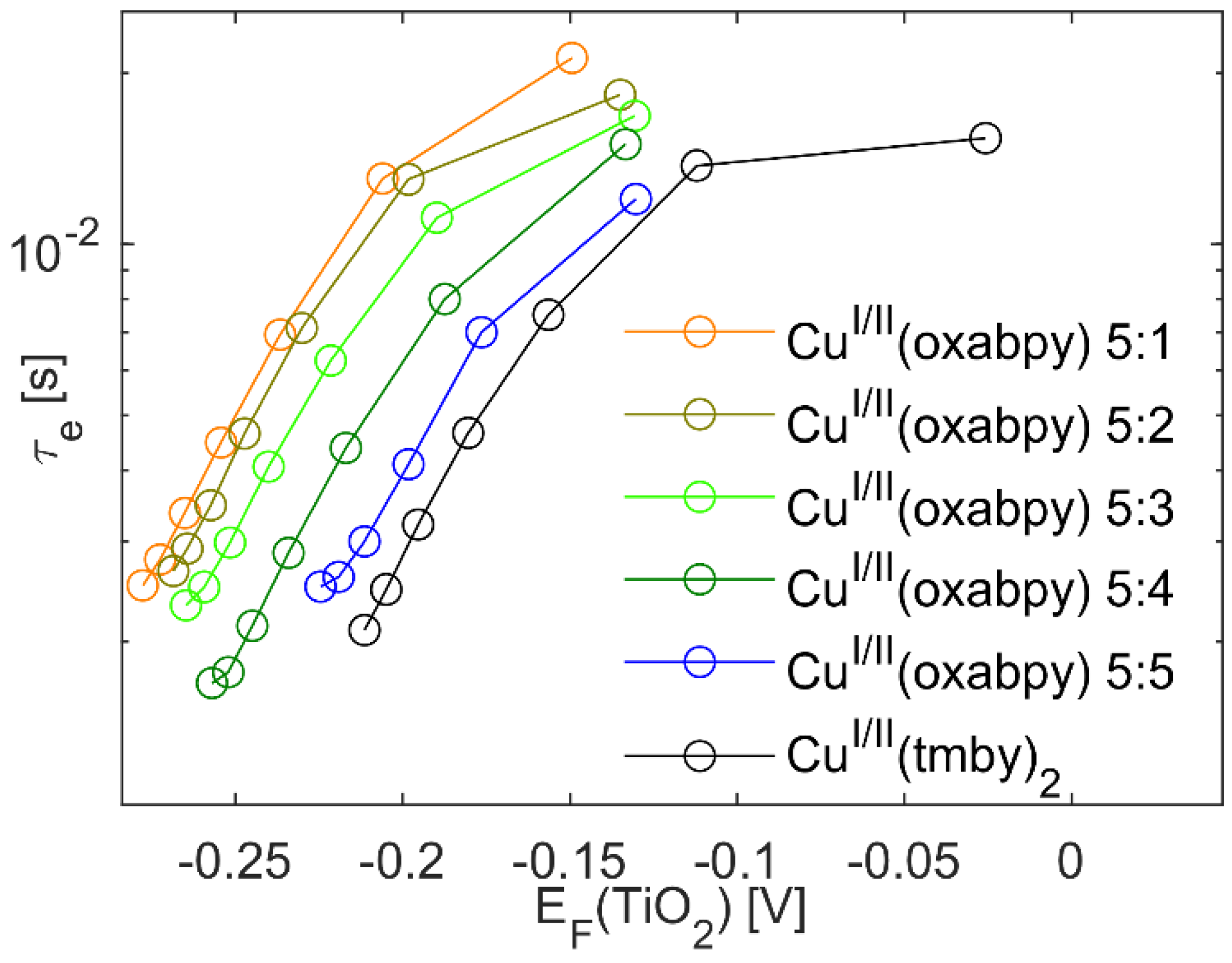
2.4. Electron Lifetime Measurements
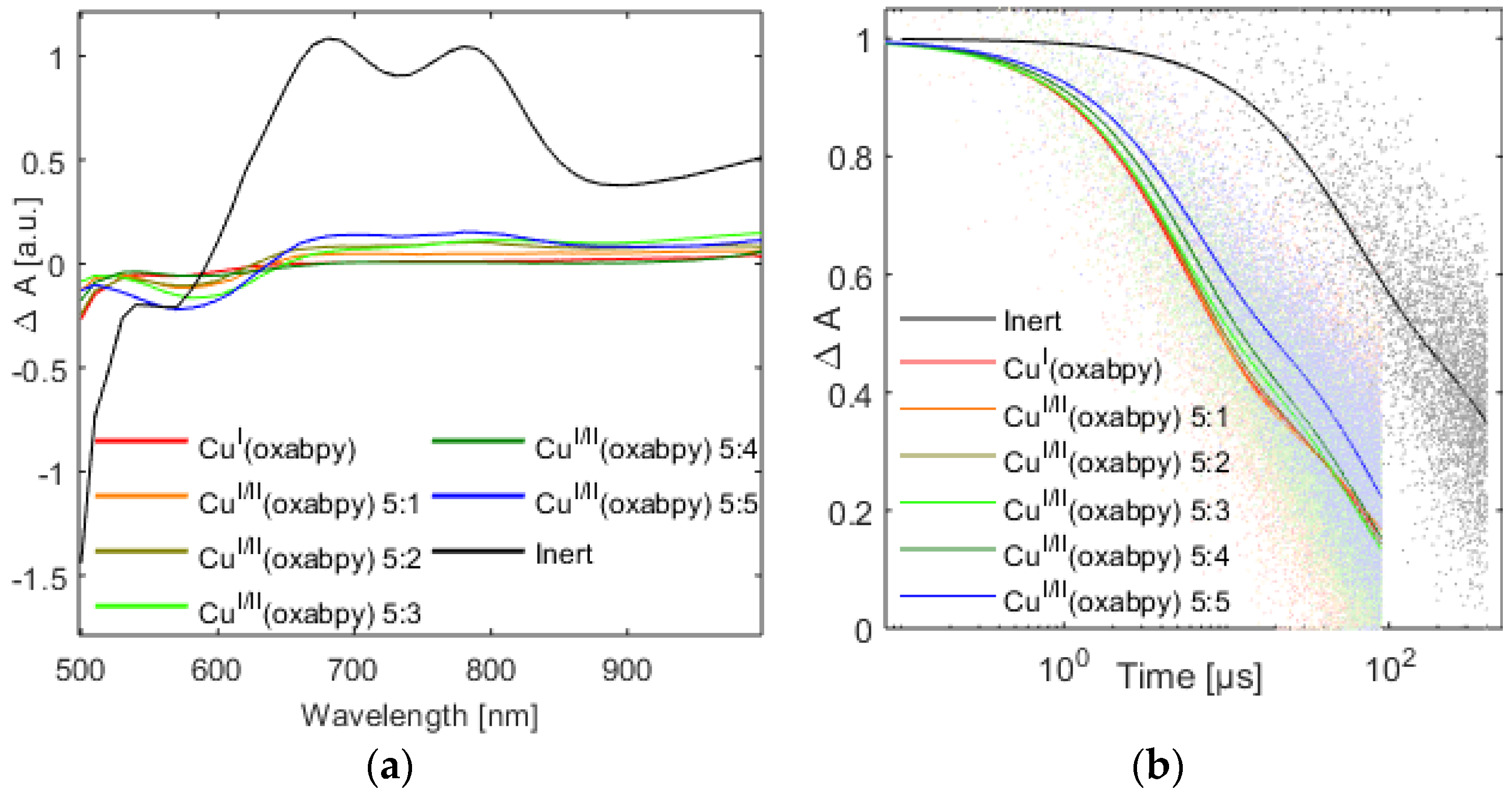
2.5. Dye Regeneration
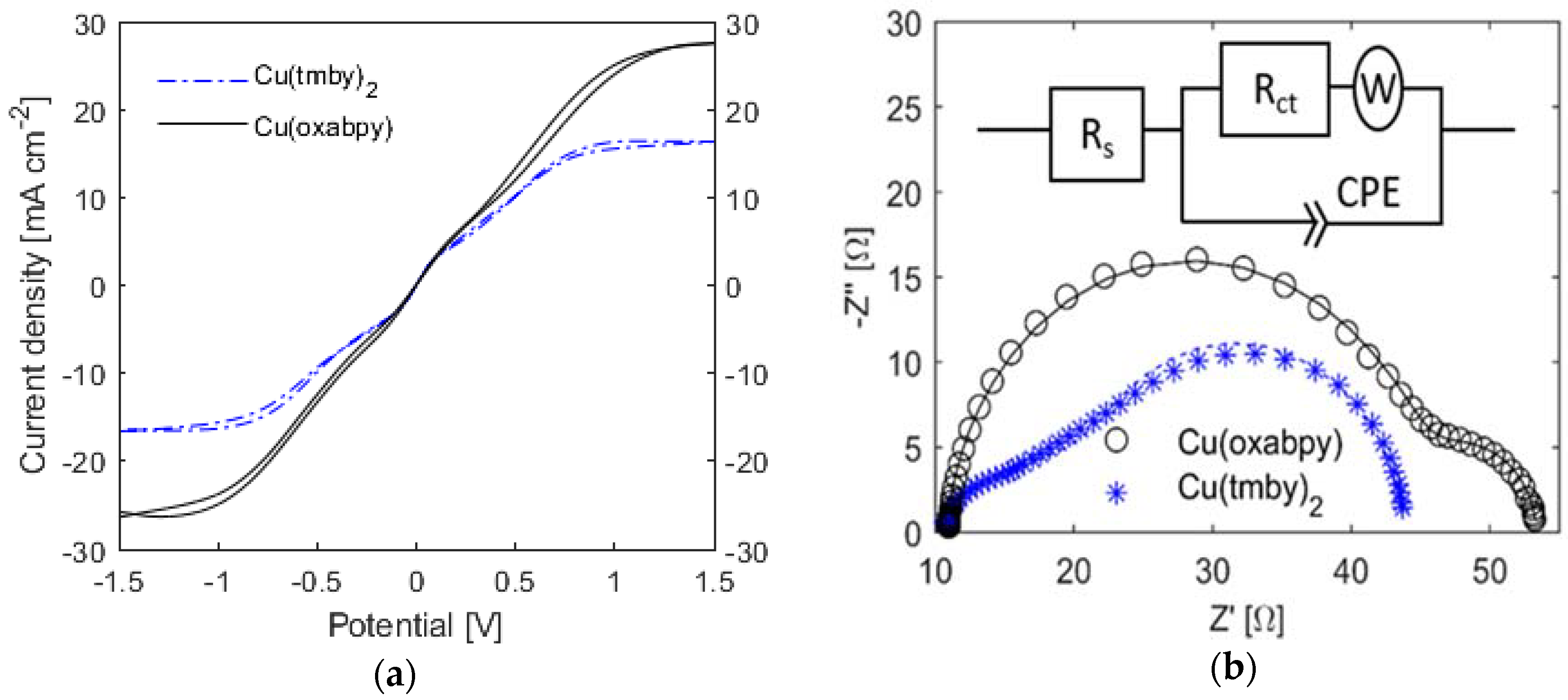
2.6. Charge Transport in the Cu(oxabpy) Redox Electrolyte
3. Materials and Methods
3.1. Materials
3.2. Synthesis of (6,6′-bis(4-(S)-isopropyl-2-oxazolinyl)-2,2′-bipyridine)Copper(II) bis (bis(trifluoromethanesulfonyl)imide) (CuII(oxabpy))
3.3. Synthesis of (6,6′-bis(4-(S)-isopropyl-2-oxazolinyl)-2,2′-bipyridine)Copper(I) bis(trifluoromethanesulfonyl)imide (CuI(oxabpy))
3.4. UV/VIS Spectroscopy
3.5. Viscosity Measurements
3.6. Raman Spectroscopy
3.7. Solar Cell Fabrication
3.8. Solar Cell Characterization
3.9. Incident Photon-to-Current Conversion Efficiency (IPCE)
3.10. Electrochemistry
3.11. Electron Lifetime Measurements
3.12. Photoinduced Absorption Spectroscopy (PIA)
3.13. Transient Absorption Spectroscopy (TAS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Gerischer, H. The impact of semiconductors on the concepts of electrochemistry. Electrochim. Acta 1990, 35, 1677–1699. [Google Scholar] [CrossRef]
- O’Regan, B.; Gratzel, M. A Low-Cost, High-Efficiency Solar-Cell Based on Dye-Sensitized Colloidal TiO2 Films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Asim, N.; Sopian, K.; Ahmadi, S.; Saeedfar, K.; Alghoul, M.A.; Saadatian, O.; Zaidi, S.H. A review on the role of materials science in solar cells. Renew. Sustain. Energy Rev. 2012, 16, 5834–5847. [Google Scholar] [CrossRef]
- Gong, J.; Liang, J.; Sumathy, K. Review on dye-sensitized solar cells (DSSCs): Fundamental concepts and novel materials. Renew. Sustain. Energy Rev. 2012, 16, 5848–5860. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Grätzel, M. Molecular photovoltaics. Acc. Chem. Res. 2000, 33, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef] [PubMed]
- Boschloo, G.; Hagfeldt, A.; Spectus, C.O.N. Characteristics of the Iodide/Triiodide Redox Mediator in Dye-Sensitized Solar Cells. Acc. Chem. Res. 2009, 42, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Privalov, T.; Boschloo, G.; Hagfeldt, A.; Svensson, P.H.; Kloo, L. A study of the interactions between I−/I3− redox mediators and organometallic sensitizing dyes in solar cells. J. Phys. Chem. C 2009, 113, 783–790. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; De Angelis, F.; Fantacci, S.; Selloni, A.; Viscardi, G.; Liska, P.; Ito, S.; Takeru, B.; Grätzel, M. Combined experimental and DFT-TDDFT computational study of photoelectrochemical cell ruthenium sensitizers. J. Am. Chem. Soc. 2005, 127, 16835–16847. [Google Scholar] [CrossRef] [PubMed]
- Feldt, S.M.; Wang, G.; Boschloo, G.; Hagfeldt, A. Effects of Driving Forces for Recombination and Regeneration on the Photovoltaic Performance of Dye-Sensitized Solar Cells using Cobalt Polypyridine Redox Couples. J. Phys. Chem. C 2011, 115, 21500–21507. [Google Scholar] [CrossRef]
- Feldt, S.M.; Gibson, E.A.; Gabrielsson, E.; Sun, L.; Boschloo, G.; Hagfeldt, A. Design of Organic Dyes and Cobalt Polypyridine Redox Mediators for High-Efficiency Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2010, 132, 16714–16724. [Google Scholar] [CrossRef] [PubMed]
- Sapp, S.A.; Elliott, C.M.; Contado, C.; Caramori, S.; Bignozzi, C.A. Substituted Polypyridine Complexes of Cobalt(II/III) as Efficient Electron-Transfer Mediators in Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2002, 124, 11215–11222. [Google Scholar] [CrossRef] [PubMed]
- Klahr, B.M.; Hamann, T.W. Performance enhancement and limitations of cobalt bipyridyl redox shuttles in dye-sensitized solar cells. J. Phys. Chem. C 2009, 113, 14040–14045. [Google Scholar] [CrossRef]
- Nelson, J.J.; Amick, T.J.; Elliott, C.M. Mass Transport of Polypyridyl Cobalt Complexes in Dye-Sensitized Solar Cells with Mesoporous TiO Photoanodes Mass Transport of Polypyridyl Cobalt Complexes in Dye-Sensitized Solar Cells with Mesoporous TiO2 Photoanodes. J. Phys. Chem. C 2008, 112, 18255–18263. [Google Scholar] [CrossRef]
- Cameron, P.J.; Peter, L.M.; Zakeeruddin, S.M.; Grätzel, M. Electrochemical studies of the Co(III)/Co(II)(dbbip)2redox couple as a mediator for dye-sensitized nanocrystalline solar cells. Coord. Chem. Rev. 2004, 248, 1447–1453. [Google Scholar] [CrossRef]
- Hattori, S.; Wada, Y.; Yanagida, S.; Fukuzumi, S. Blue copper model complexes with distorted tetragonal geometry acting as effective electron-transfer mediators in dye-sensitized solar cells. J. Am. Chem. Soc. 2005, 127, 9648–9654. [Google Scholar] [CrossRef] [PubMed]
- Freitag, M.; Giordano, F.; Yang, W.; Pazoki, M.; Hao, Y.; Zietz, B.; Grätzel, M.; Hagfeldt, A.; Boschloo, G. Copper Phenanthroline as a Fast and High-Performance Redox Mediator for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2016, 120, 9595–9603. [Google Scholar] [CrossRef]
- Bai, Y.; Yu, Q.; Cai, N.; Wang, Y.; Zhang, M.; Wang, P. High-efficiency organic dye-sensitized mesoscopic solar cells with a copper redox shuttle. Chem. Commun. 2011, 47, 4376–4378. [Google Scholar] [CrossRef] [PubMed]
- Saygili, Y.; Söderberg, M.; Pellet, N.; Giordano, F.; Cao, Y.; Munoz-García, A.B.; Zakeeruddin, S.M.; Vlachopoulos, N.; Pavone, M.; Boschloo, G.; et al. Copper Bipyridyl Redox Mediators for Dye-Sensitized Solar Cells with High Photovoltage. J. Am. Chem. Soc. 2016, 138, 15087–15096. [Google Scholar] [CrossRef] [PubMed]
- Freitag, M.; Teuscher, J.; Saygili, Y.; Zhang, X.; Giordano, F.; Liska, P.; Hua, J.; Zakeeruddin, S.M.; Moser, J.E.; Grätzel, M.; et al. Dye-sensitized solar cells for efficient power generation under ambient lighting. Nat. Photonics 2017, 11, 372–378. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, Y.; Bahng, H.W.; Cao, Y.; Yi, C.; Saygili, Y.; Luo, J.; Liu, Y.; Kavan, L.; Moser, J.E.; et al. Comprehensive control of voltage loss enables 11.7% efficient solid-state dye-sensitized solar cells. Energy Environ. Sci. 2018, 1–9. [Google Scholar] [CrossRef]
- Flasque, M.; Van Nhien, A.N.; Swiatowska, J.; Seyeux, A.; Davoisne, C.; Sauvage, F. Interface Stability of a TiO2/3-Methoxypropionitrile-Based Electrolyte: First Evidence for Solid Electrolyte Interphase Formation and Implications. ChemPhysChem 2014, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.W.; Duffy, N.W.; Wilson, G.J. Efficient all-printable solid-state dye-sensitized solar cell based on a low-resistivity carbon composite counter electrode and highly doped hole transport material. J. Phys. Chem. C 2015, 119, 11410–11418. [Google Scholar] [CrossRef]
- Freitag, M.; Daniel, Q.; Pazoki, M.; Sveinbjörnsson, K.; Zhang, J.; Sun, L.; Hagfeldt, A.; Boschloo, G. High-efficiency dye-sensitized solar cells with molecular copper phenanthroline as solid hole conductor. Energy Environ. Sci. 2015, 8, 2634–2637. [Google Scholar] [CrossRef]
- Mosconi, E.; Yum, J.-H.; Kessler, F.; Gómez García, C.J.; Zuccaccia, C.; Cinti, A.; Nazeeruddin, M.K.; Grätzel, M.; De Angelis, F. Cobalt Electrolyte/Dye Interactions in Dye-Sensitized Solar Cells: A Combined Computational and Experimental Study. J. Am. Chem. Soc. 2012, 134, 19438–19453. [Google Scholar] [CrossRef] [PubMed]
- Yum, J.-H.; Baranoff, E.; Kessler, F.; Moehl, T.; Ahmad, S.; Bessho, T.; Marchioro, A.; Ghadiri, E.; Moser, J.-E.; Yi, C.; et al. A cobalt complex redox shuttle for dye-sensitized solar cells with high open-circuit potentials. Nat. Commun. 2012, 3, 631. [Google Scholar] [CrossRef] [PubMed]
- Feldt, S.M.; Lohse, P.W.; Kessler, F.; Nazeeruddin, M.K.; Grätzel, M.; Boschloo, G.; Hagfeldt, A. Regeneration and recombination kinetics in cobalt polypyridine based dye-sensitized solar cells, explained using Marcus theory. Phys. Chem. Chem. Phys. 2013, 15, 7087. [Google Scholar] [CrossRef] [PubMed]
- Kivelson, D.; Neiman, R. ESR Studies on the Bonding in Copper Complexes. J. Chem. Phys. 1961, 35, 149–155. [Google Scholar] [CrossRef]
- Billig, E.; Williams, R.; Bernal, I.; Waters, J.H.; Gray, H.B. The Electronic Structures of Square-Planar Metal Complexes. II. The Complexes of Maleonitriledithiolate with Copper(II), Nickel(II), Palladium(II), and Platinum(II). Inorg. Chem. 1964, 3, 663–666. [Google Scholar] [CrossRef]
- Freitag, M.; Yang, W.; Fredin, L.A.; D’Amario, L.; Karlsson, K.M.; Hagfeldt, A.; Boschloo, G. Supramolecular Hemicage Cobalt Mediators for Dye-Sensitized Solar Cells. ChemPhysChem 2016, 17, 3845–3852. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.M.; Qiu, Z.; Häggman, L.; Mirmohades, M.; Johansson, M.B.; Edvinsson, T.; Boschloo, G. Frustrated Lewis pair-mediated recrystallization of CH3NH3PbI3 for improved optoelectronic quality and high voltage planar perovskite solar cells. Energy Environ. Sci. 2016, 9, 3770–3782. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision A. 03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D.; Johnson, E.R. A simple effective potential for exchange. J. Chem. Phys. 2006, 124. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef] [PubMed]
- Scalmani, G.; Frisch, M.J.; Mennucci, B.; Tomasi, J.; Cammi, R.; Barone, V. Geometries and properties of excited states in the gas phase and in solution: Theory and application of a time-dependent density functional theory polarizable continuum model. J. Chem. Phys. 2006, 124. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Hao, Y.; Vlachopoulos, N.; Eriksson, A.I.K.; Boschloo, G. Studies on the Interfacial Electric Field and Stark Effect at the TiO2/Dye/Electrolyte Interface. J. Phys. Chem. C 2016, 120, 22215–22224. [Google Scholar] [CrossRef]
- Cappel, U.B.; Feldt, S.M.; Schöneboom, J.; Hagfeldt, A.; Boschloo, G. The Influence of Local Electric Fields on Photoinduced Absorption in Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2010, 132, 9096–9101. [Google Scholar] [CrossRef] [PubMed]
- Ferdowsi, P.; Saygili, Y.; Zakeeruddin, S.M.; Mokhtari, J.; Grätzel, M.; Hagfeldt, A.; Kavan, L. Alternative bases to 4-tert-butylpyridine for dye-sensitized solar cells employing copper redox mediator. Electrochim. Acta 2018, 265, 194–201. [Google Scholar] [CrossRef]
- Kavan, L.; Krysova, H.; Janda, P.; Tarabkova, H.; Saygili, Y.; Freitag, M.; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. Novel highly active Pt/graphene catalyst for cathodes of Cu(II/I)-mediated dye-sensitized solar cells. Electrochim. Acta 2017, 251, 167–175. [Google Scholar] [CrossRef]
- Cao, Y.; Saygili, Y.; Ummadisingu, A.; Teuscher, J.; Luo, J.; Pellet, N.; Giordano, F.; Zakeeruddin, S.M.; Moser, J.-E.; Freitag, M.; et al. 11% efficiency solid-state dye-sensitized solar cells with copper(II/I) hole transport materials. Nat. Commun. 2017, 8, 15390. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Gorlov, M.; Nissfolk, J.; Boschloo, G.; Kloo, L. Investigation of Iodine Concentration Effects in Electrolytes for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2010, 114, 10612–10620. [Google Scholar] [CrossRef]
- Peng, B.; Jungmann, G.; Jäger, C.; Haarer, D.; Schmidt, H.-W.; Thelakkat, M. Systematic investigation of the role of compact TiO2 layer in solid state dye-sensitized TiO2 solar cells. Coord. Chem. Rev. 2004, 248, 1479–1489. [Google Scholar] [CrossRef]
- Ellis, H.; Vlachopoulos, N.; Häggman, L.; Perruchot, C.; Jouini, M.; Boschloo, G.; Hagfeldt, A. PEDOT counter electrodes for dye-sensitized solar cells prepared by aqueous micellar electrodeposition. Electrochim. Acta 2013, 107, 45–51. [Google Scholar] [CrossRef]
- Solar Spectral Irradiance: Air Mass. Available online: http://rredc.nrel.gov/solar/spectra/am1.5/ (accessed on 28 March 2018).

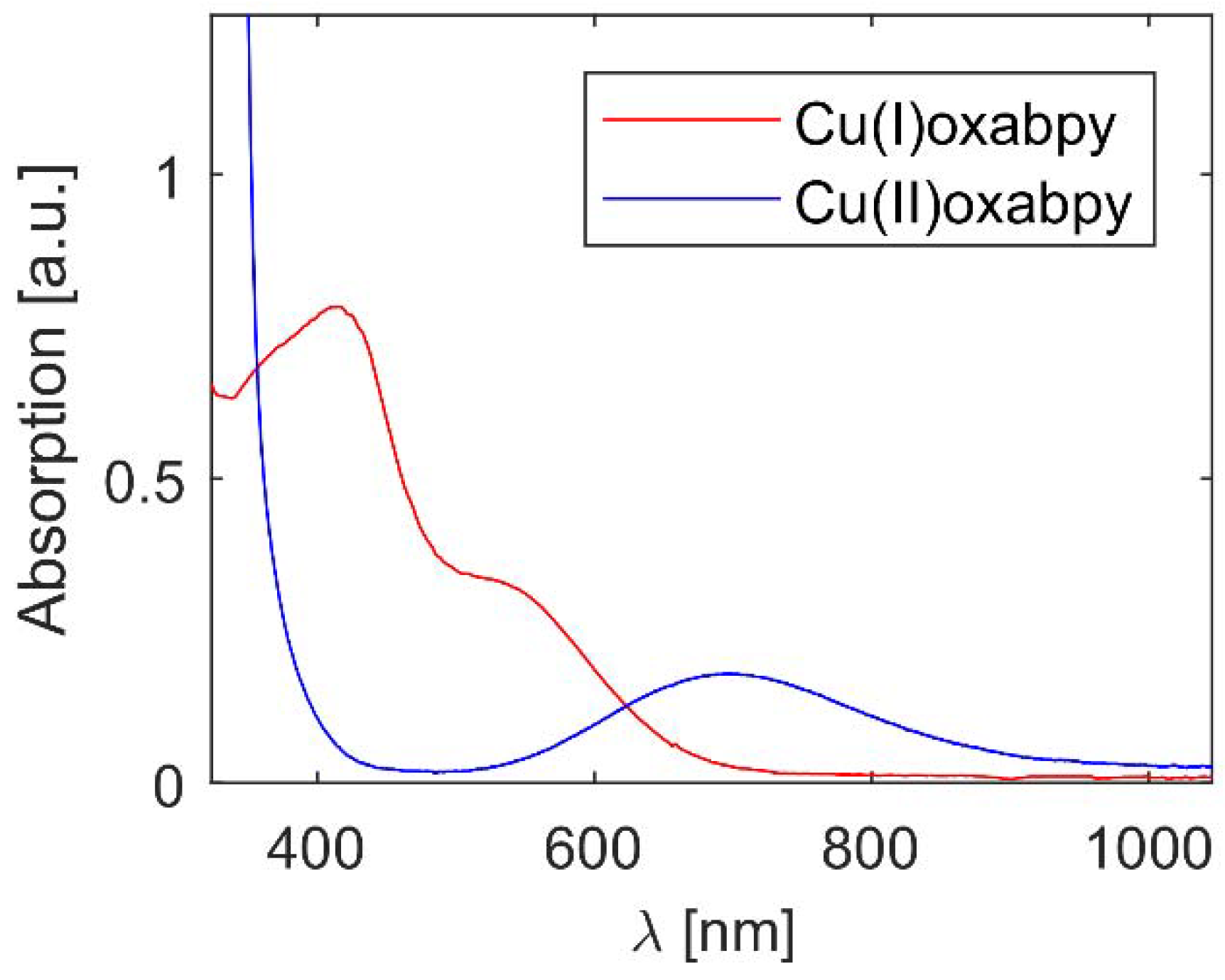

| Compound | Eredox (V vs. NHE) | λmax,abs (nm) | ε (M−1 cm−1) |
|---|---|---|---|
| CuI(oxabpy)TFSI | 0.66 * | 415 | 1587 |
| CuII(oxabpy)TFSI2 | 697 | 93 |
| Electrolyte | Voc (mV) | Jsc (mA·cm−2) | Fill Factor | PCE (%) |
|---|---|---|---|---|
| Cu(tmby)2 | 1040 | 10.5 | 0.71 | 7.8 |
| 10% light | 875 | 1.44 | 0.78 | 10.0 |
| Cu(oxabpy) | 920 | 9.75 | 0.69 | 6.2 |
| 10% light | 855 | 1.32 | 0.79 | 8.9 |
| τ1/2 (µs) | φreg (%) | |
|---|---|---|
| Inert | 104 | - |
| CuI(oxabpy) | 6.19 | 94.0 |
| CuI/II(oxabpy) 5:1 | 6.33 | 93.9 |
| CuI/II(oxabpy) 5:2 | 6.70 | 93.5 |
| CuI/II(oxabpy) 5:3 | 7.39 | 92.9 |
| CuI/II(oxabpy) 5:4 | 8.52 | 91.8 |
| CuI/II(oxabpy) 5:5 | 12.6 | 87.9 |
| Cu(tmby)2 | Cu(oxabpy) | |
|---|---|---|
| JL (mA cm−2) | 16.6 | 27.5 |
| D (10−6 cm2 s−1) for CuII from JL | 8.6 (10; 12) [41,42] | 14.3 |
| RS (Ω) | 10.5 | 10.8 |
| RCT (Ω) | 6.92 | 33.4 |
| CPE: Q (10−3 Ω−1 s−β) | 0.337 | 0.124 |
| CPE: β | 0.847 | 0.967 |
| W: Rw (Ω) | 26.5 | 9.09 |
| W: Tw (s) | 0.208 | 0.285 |
| D (10−6 cm2 s−1) for CuII from Rw | 22.4 (22; 26) [20,41] | 63.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michaels, H.; Benesperi, I.; Edvinsson, T.; Muñoz-Garcia, A.B.; Pavone, M.; Boschloo, G.; Freitag, M. Copper Complexes with Tetradentate Ligands for Enhanced Charge Transport in Dye-Sensitized Solar Cells. Inorganics 2018, 6, 53. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics6020053
Michaels H, Benesperi I, Edvinsson T, Muñoz-Garcia AB, Pavone M, Boschloo G, Freitag M. Copper Complexes with Tetradentate Ligands for Enhanced Charge Transport in Dye-Sensitized Solar Cells. Inorganics. 2018; 6(2):53. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics6020053
Chicago/Turabian StyleMichaels, Hannes, Iacopo Benesperi, Tomas Edvinsson, Ana Belén Muñoz-Garcia, Michele Pavone, Gerrit Boschloo, and Marina Freitag. 2018. "Copper Complexes with Tetradentate Ligands for Enhanced Charge Transport in Dye-Sensitized Solar Cells" Inorganics 6, no. 2: 53. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics6020053