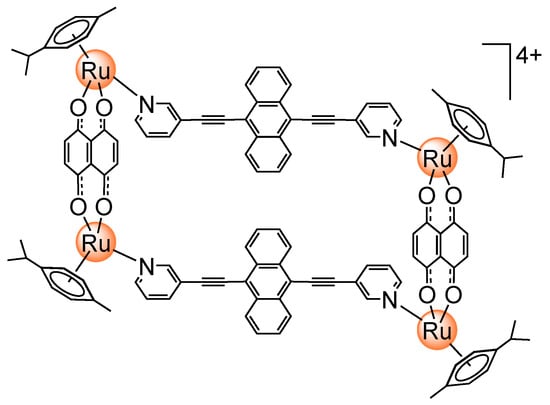
Arene Ruthenium Metalla-Assemblies with Anthracene Moieties for PDT Applications
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. Material and Methods
3.2. Synthesis and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zuluaga, M.F.; Lange, N. Combination of photodynamic therapy with anti-cancer agents. Curr. Med. Chem. 2008, 15, 1655–1673. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Usuda, J.; Kato, H.; Okunaka, T.; Furukawa, K.; Tsutsui, H.; Yamada, K.; Suga, Y.; Honda, H.; Nagatsuka, Y.; Ohira, T.; et al. Photodynamic therapy (PDT) for lung cancers. J. Thorac. Oncol. 2006, 1, 489–493. [Google Scholar] [CrossRef]
- Baron, E.D.; Malbasa, C.L.; Santo-Domingo, D.; Fu, P.; Miller, J.D.; Hanneman, K.K.; Hsia, A.H.; Oleinick, N.L.; Colussi, V.C.; Cooper, K.D. Silicon phthalocyanine (Pc4) photodynamic therapy is a safe modality for cutaneous neoplasms: Results of a phase 1 clinical trial. Lasers Surg. Med. 2010, 42, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Babilas, P.; Schreml, S.; Landthaler, M.; Szeimies, R.M. Photodynamic therapy in dermatology: State-of-the-art. Photodermatol. Photoimmunol. Photomed. 2010, 26, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.Y.; Dai, T.; Hamblin, M.R. Photodynamic therapy for infections: Clinical applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.M.; Mackenzie, G.D.; Banks, M.R.; Mosse, C.A.; Haidry, R.; Green, S.; Thorpe, S.; Rodriguez-Justo, M.; Winstanley, A.; Novelli, M.R.; et al. A randomised controlled trial of ALA vs. Photofrin photodynamic therapy for high-grade dysplasia arising in Barrett’s oesophagus. Lasers Med. Sci. 2013, 28, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, V.G.; Somers, M.L. PHOTOFRIN-mediated photodynamic therapy for treatment of early stage (Tis-T2N0M0) SqCCa of oral cavity and oropharynx. Lasers Surg. Med. 2010, 42, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hsu, C.H.; Huang, C.C.; Chang, P.Y. Development of therapeutic Au-methylene blue nanoparticles for targeted photodynamic therapy of cervical cancer cells. ACS Appl. Mater. Interfaces 2015, 7, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Yavari, N.; Andersson-Engels, S.; Segersten, U.; Malmstrom, P.U. An overview on preclinical and clinical experiences with photodynamic therapy for bladder cancer. Can. J. Urol. 2011, 18, 5343–5351. [Google Scholar]
- Montazerabadi, A.R.; Sazgarnia, A.; Bahreyni-Toosi, M.H.; Ahmadi, A.; Aledavood, A. The effects of combined treatment with ionizing radiation and indocyanine green-mediated photodynamic therapy on breast cancer cells. J. Photochem. Photobiol. B 2012, 109, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Wakui, M.; Yokoyama, Y.; Wang, H.; Shigeto, T.; Futagami, M.; Mizunuma, H. Efficacy of a methyl ester of 5-aminolevulinic acid in photodynamic therapy for ovarian cancers. J. Cancer Res. Clin. Oncol. 2010, 136, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Patrice, T.; Moor, A.C.E.; Ortel, B.; Hasan, T. Mechanism of photodynamic therapy. In Photodynamic Therapy; Patrice, T., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2003; Volume 2, pp. 19–58. [Google Scholar]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part three-Photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagn. Photodyn. Ther. 2005, 2, 91–106. [Google Scholar] [CrossRef]
- Foote, C.S. Definition of type I and type II photosensitized oxidation. Photochem. Photobiol. 1991, 54, 659. [Google Scholar] [CrossRef] [PubMed]
- Debele, T.A.; Peng, S.; Tsai, H.C. Drug carrier for photodynamic cancer therapy. Int. J. Mol. Sci. 2015, 16, 22094–22136. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Wilson, W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.H.; Conger, A.D.; Ebert, M.; Hornsey, S.; Scott, O.C. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br. J. Radiol. 1953, 26, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Thomlinson, R.H.; Gray, L.H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br. J. Radiol. 1955, 9, 539–549. [Google Scholar] [CrossRef]
- Vaupel, P.; Schlenger, K.; Knoop, C.; Höckel, M. Oxygenation of human tumors: Evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991, 51, 3316–3322. [Google Scholar] [PubMed]
- Nordsmark, M.; Overgaard, M.; Overgaard, J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother. Oncol. 1996, 41, 31–39. [Google Scholar] [CrossRef]
- Brizel, D.M.; Dodge, R.K.; Clough, R.W.; Dewhirst, M.W. Oxygenation of head and neck cancer: Changes during radiotherapy and impact on treatment outcome. Radiother. Oncol. 1999, 53, 113–117. [Google Scholar] [CrossRef]
- Durand, R.E. Keynote address: The influence of microenvironmental factors on the activity of radiation and drugs. Int. J. Radiat. Oncol. Biol. Phys. 1991, 20, 253–258. [Google Scholar] [CrossRef]
- Batchelder, R.M.; Wilson, W.R.; Hay, M.P.; Denny, W.A. Oxygen dependence of the cytotoxicity of the enediyne anti-tumour antibiotic esperamicin A1. Br. J. Cancer Suppl. 1996, 27, S52–S56. [Google Scholar] [PubMed]
- Teicher, B.A.; Lazo, J.S.; Sartorelli, A.C. Classification of antineoplastic agents by their selective classification of antineoplastic agents by their selective toxicities toward oxygenated and hypoxic tumor cells. Cancer Res. 1981, 41, 73–81. [Google Scholar] [PubMed]
- Mizukami, Y.; Li, J.; Zhang, X.; Zimmer, M.A.; Iliopoulos, O.; Chung, D.C. Hypoxia-inducible factor-1-independent regulation of vascular endothelial growth factor by hypoxia in colon cancer. Cancer Res. 2004, 64, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Wartenberg, M.; Ling, F.C.; Müschen, M.; Klein, F.; Acker, H.; Gassmann, M.; Petrat, K.; Pütz, V.; Hescheler, J.; Sauer, H. Regulation of the multidrug resistance transporter P-glycoprotein in multicellular tumor spheroids by hypoxia-inducible factor (HIF-1) and reactive oxygen species. FASEB J. 2003, 17, 503–505. [Google Scholar] [CrossRef] [PubMed]
- Graeber, T.G.; Osmanian, C.; Jacks, T.; Housman, D.E.; Koch, C.J.; Lowe, S.W.; Giaccia, A.J. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 1996, 379, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Erler, J.T.; Cawthorne, C.W.; Williams, K.J.; Koritzinsky, M.; Wouters, B.G.; Wilson, C.; Miler, C.; Demonacos, C.; Stratford, I.J.; Dive, C. Hypoxia-mediated down-regulation of Bid and Bax in tumors occurs via hypoxia-inducible factor 1-dependent and -independent mechanisms and contributes to drug resistance. Mol. Cell. Biol. 2004, 24, 2875–2889. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Glazer, P.M. Mutagenesis induced by the tumor microenvironment. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1998, 400, 439–446. [Google Scholar] [CrossRef]
- Harris, A.L. Hypoxia—a key regulatory factor in tumour growth. Nat. Rev. Cancer 2002, 2, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Rouschop, K.M.A.; van den Beucken, T.; Dubois, L.; Niessen, H.; Bussink, J.; Savelkouls, K.; Keulers, T.; Mujcic, H.; Landuyt, W.; Voncken, J.W.; et al. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J. Clin. Investing. 2010, 120, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ohh, M. Oxygen-mediated endocytosis in cancer. J. Cell. Mol. Med. 2010, 14, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit. Rev. Biochem. Mol. Biol. 2000, 35, 71–103. [Google Scholar] [CrossRef] [PubMed]
- Kioi, M.; Vogel, H.; Schultz, G.; Hoffman, R.M.; Harsh, G.R.; Brown, J.M. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J. Clin. Investing. 2010, 120, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Therrien, B. Transporting and shielding photosensitizers by using water-soluble organometallic cages: A new strategy in drug delivery and photodynamic therapy. Chem. Eur. J. 2013, 19, 8378–8386. [Google Scholar] [CrossRef] [PubMed]
- Mari, C.; Pierroz, V.; Ferrari, S.; Gasser, G. Combination of Ru(II) complexes and light: New frontiers in cancer therapy. Chem. Sci. 2015, 6, 2660–2686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Bole, Y.; Zhang, P.; Huang, J.; Chen, Y.; Gasser, G.; Ji, L.; Chao, H. Highly charged ruthenium(II) polypyridyl complexes as lysosome-localized photosensitizers for two-photon photodynamic therapy. Angew. Chem. Int. Ed. 2015, 54, 14049–14052. [Google Scholar] [CrossRef] [PubMed]
- Pierroz, V.; Rubbiani, R.; Gentili, C.; Patra, M.; Mari, C.; Gasser, G.; Ferrari, S. Dual mode of cell death upon the photo-irradiation if a Ru(II) polypyridyl complex in interphase or mitosis. Chem. Sci. 2016, 7, 6115–6124. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.; Huang, H.; Kaiser, A.; Pierroz, V.; Blacque, O.; Chao, H.; Gasser, G. Evaluation of the medicinal potential of two ruthenium(II) polypyridine complexes as one- and two-photon photodynamic therapy photosensitizers. Chem. Eur. J. 2017, 23, 9888–9896. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, F.; Karges, J.; Gasser, G. Critical overview of the use of Ru(II) polypyridyl complexes as photosensitizers in one-photon and two-photon photodynamic therapy. Acc. Chem. Res. 2017, 50, 2727–2736. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, C.; Rees, T.W.; Ke, L.; Ji, L.; Chao, H. Harnessing ruthenium(II) as photodynamic agents: Encouraging advances in cancer therapy. Coord. Chem. Rev. 2018, 363, 17–28. [Google Scholar] [CrossRef]
- Alessio, E.; Mestroni, G.; Bergamo, A.; Sava, G. Ruthenium antimetastatic agents. Curr. Top. Med. Chem. 2004, 4, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Ang, W.H.; Dyson, P.J. Classical and non-classical ruthenium-based anticancer drugs: Towards targeted chemotherapy. Eur. J. Inorg. Chem. 2006, 2006, 4003–4018. [Google Scholar] [CrossRef]
- Bergamo, A.; Sava, G. Ruthenium complexes can target determinants of tumors malignancy. Dalton Trans. 2007, 13, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, C.G.; Dyson, P.J. Bioorganometallic chemistry—From teaching paradigms to medicinal applications. Chem. Soc. Rev. 2009, 38, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Bruijnincx, P.C.A.; Sadler, P.J. New trends for metal complexes with anticancer activity. Curr. Opin. Chem. Biol. 2008, 12, 197–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meggers, E.; Atilla-Gokcumen, G.E.; Bregman, H.; Maksimoska, J.; Mulcahy, S.P.; Pagano, N.; Williams, D.S. Exploring chemical space with organometallics: Ruthenium complexes as protein kinase inhibitors. Synlett 2007, 8, 1177–1189. [Google Scholar] [CrossRef]
- Boca, S.C.; Four, M.; Bonn, A.; van der Sanden, B.; Astilean, S.; Baldec, P.L.; Lemercier, G. An ethylene-glycol decorated ruthenium(II) complex for two-photon photodynamic therapy. Chem. Commun. 2009, 0, 4590–4592. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R. Photosensitized generation of singlet oxygen. Photochem. Photobiol. 2006, 82, 1161–1177. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, F.; Govindaswamy, P.; Süss-Fink, G.; Ang, W.H.; Dyson, P.J.; Juillerat-Jeanneret, L.; Therrien, B. Ruthenium porphyrin compounds for photodynamic therapy of cancer. J. Med. Chem. 2008, 51, 1811–1816. [Google Scholar] [CrossRef] [PubMed]
- Pernot, M.; Bastogne, T.; Barry, N.P.E.; Therrien, B.; Koellensperger, G.; Hann, S.; Reshetov, V.; Barberi-Heyob, M. Systems biology approach for in vivo photodynamic therapy optimization of ruthenium-porphyrin compounds. J. Photochem. Photobiol. B 2012, 117, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, F.; Govindaswamy, P.; Zava, O.; Süss-Fink, G.; Juillerat-Jeanneret, L.; Therrien, B. Combined arene ruthenium porphyrins as chemotherapeutics and photosensitizers for cancer therapy. J. Biol. Inorg. Chem. 2009, 14, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Freudenreich, J.; Barry, N.P.E.; Süss-Fink, G.; Therrien, B. Permanent encapsulation or host–guest behavior of aromatic molecules in hexanuclear arene ruthenium prisms. Eur. J. Inorg. Chem. 2010, 2010, 2400–2405. [Google Scholar] [CrossRef]
- Freudenreich, J.; Furrer, J.; Süss-Fink, G.; Therrien, B. Template-directed synthesis of hexanuclear arene ruthenium complexes with trigonal-prismatic architecture based on 2,4,6-tris(3-pyridyl)triazine ligands. Organometallics 2011, 30, 942–951. [Google Scholar] [CrossRef]
- Freudenreich, J.; Dalvit, C.; Süss-Fink, G.; Therrien, B. Encapsulation of photosensitizers in hexa- and octanuclear organometallic cages: Synthesis and characterization of carceplex and host−guest systems in solution. Organometallics 2013, 32, 3018–3033. [Google Scholar] [CrossRef]
- Schmitt, F.; Freudenreich, J.; Barry, N.P.E.; Juillerat-Jeanneret, L.; Süss-Fink, G.; Therrien, B. Organometallic cages as vehicles for intracellular release of photosensitizers. J. Am. Chem. Soc. 2012, 134, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Cobo, S.; Lafolet, F.; Saint-Aman, E.; Philouze, C.; Bucher, C.; Silvi, S.; Credi, A.; Royal, G. Reactivity of a pyridinium-substituted dimethyldihydropyrene switch under aerobic conditions: Self-sensitized photo-oxygenation and thermal release of singlet oxygen. Chem. Comm. 2015, 51, 13886–13889. [Google Scholar] [CrossRef] [PubMed]
- Benz, S.; Nötzli, S.; Siegel, J.S.; Eberli, D.; Jessen, H.J. Controlled oxygen release from pyridone endoperoxides promotes cell survival under anoxic conditions. J. Med. Chem. 2013, 56, 10171–10182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klaper, M.; Linker, T. Evidence for an oxygen anthracene sandwich complex. Angew. Chem. Int. Ed. 2013, 52, 11896–11899. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Xu, S.; Wang, P.; Hu, X.X.; Zhang, J.; Yuan, L.; Zhang, X.B.; Tan, W. An efficient two-photon fluorescent probe for monitoring mitochondrial singlet oxygen in tissues during photodynamic therapy. Chem. Commun. 2016, 52, 12330–12333. [Google Scholar] [CrossRef] [PubMed]
- Asadirad, A.M.; Erno, Z.; Branda, N.R. Photothermal release of singlet oxygen from gold nanoparticles. Chem. Commun. 2013, 49, 5639–5641. [Google Scholar] [CrossRef] [PubMed]
- Aubry, J.M.; Pierlot, C.; Rigaudy, J.; Schmidt, R. Reversible binding of oxygen to aromatic compounds. Acc. Chem. Res. 2003, 36, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, A.; Piot, L.; Samorì, P.; Jouaiti, A.; Hosseini, M.W. Molecular tectonics at the solid/liquid interface: Controlling of 1D coordination networks on graphite surfaces. Adv. Mater. 2009, 21, 1131–1136. [Google Scholar] [CrossRef]
- Yan, H.; Süss-Fink, G.; Neels, A.; Stoeckli-Evans, H. Mono-, di- and tetra-nuclear p-cymene ruthenium complexes containing oxalato ligands. J. Chem. Soc. Dalton Trans. 1997, 1997, 4345–4350. [Google Scholar] [CrossRef]
- Therrien, B.; Süss-Fink, G.; Govindaswamy, P.; Renfrew, A.K.; Dyson, P.J. The “complex-in-a-complex” cations [(acac)2M⊂Ru6(p-iPrC6H4Me)6(tpt)2(dhbq)3]6+: A trojan horse for cancer cells. Angew. Chem. Int. Ed. Engl. 2008, 47, 3773–3776. [Google Scholar] [CrossRef] [PubMed]
- Barry, N.P.E.; Therrien, B. Host–Guest Chemistry in the Hexanuclear (Arene)ruthenium Metalla-Prismatic Cage [Ru6(p-cymene)6(tpt)2(dhnq)3]6+. Eur. J. Inorg. Chem. 2009, 2009, 4695–4700. [Google Scholar] [CrossRef]
- Therrien, B. Arene Ruthenium Cages: Boxes Full of Surprises. Eur. J. Inorg. Chem. 2009, 2009, 2445–2453. [Google Scholar] [CrossRef] [Green Version]
- Barry, N.; Furrer, J.; Therrien, B. In- and Out-of-Cavity Interactions by Modulating the Size of Ruthenium Metallarectangles. Helv. Chim. Acta 2010, 93, 1313–1328. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.S., Jr. Diffusion ordered nuclear magnetic resonance spectroscopy: Principles and applications. Prog. Nucl. Magn. Reson. Spectrosc. 1999, 34, 203–256. [Google Scholar] [CrossRef]
- Barry, N.P.E.; Furrer, J.; Freudenreich, J.; Süss-Fink, G.; Therrien, B. Designing the host-guest properties of tetranuclear arene ruthenium metalla-rectangles to accommodate a pyrene molecule. Eur. J. Inorg. Chem. 2010, 5, 725–728. [Google Scholar] [CrossRef]
- Tidmarsh, I.S.; Taylor, B.F.; Hardie, M.J.; Russo, L.; Clegg, W.; Ward, M.D. Further investigations into tetrahedral M4L6 cage complexes containing guest anions: New structures and NMR spectroscopic studies. New J. Chem. 2009, 33, 366–375. [Google Scholar] [CrossRef]
- Furrer, M.A.; Schmitt, F.; Wiederkehr, M.; Juillerat-Jeanneret, L.; Therrien, B. Cellular delivery of pyrenyl-arene ruthenium complexes by a water-soluble arene ruthenium metalla-cage. Dalton Trans. 2012, 41, 7201–7211. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Elumalai, P.; Singh, N.; Kim, H.; Lee, S.U.; Chi, K.W. Selective synthesis of ruthenium(II) metalla[2]-catenane via solvent and guest-dependent self-assembly. J. Am. Chem. Soc. 2015, 137, 4674–4677. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Uribe, C.E.; Vallejo-Lozada, W.A.; Martínez-Ortega, F. Photooxidation of anthracene under visible light with metallocarboxyphenylporphyrins. Rev. Fac. Ing. Univ. Antioquia 2014, 73, 225–230. [Google Scholar]
- Alvariño Bouza, C. Autoensamblaje de nuevos metalociclos, complejos de inclusión y moléculas mecánicamenta entrelazadas. Ph.D. Thesis, Universidade da Coruña, A Coruña, Spain, 2015. [Google Scholar]
- Ware, W.R. Oxygen quenching of fluorescence in solution: An experimental study of the diffusion process. J. Phys. Chem. 1962, 66, 455–458. [Google Scholar] [CrossRef]
- Berlman, I.B.; Steingraber, O.J. Further evidence of a hidden singlet transition in biphenyl. J. Chem. Phys. 1965, 43, 2140–2141. [Google Scholar] [CrossRef]
- Lemp, E.; Zanocco, A.L. Singlet oxygen chemical acceptors. In Singlet Oxygen: Applications in Biosciences and Nanosciences; Nonell, S., Flors, C., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2016; Volume 2, pp. 83–101. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. ORTEP-3 for Windows—A version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Cryst. 1997, 30, 565. [Google Scholar] [CrossRef]








| Photosensitizers | Approved | Trial | Cancer Types |
|---|---|---|---|
| Porfimer sodium (hematoporphyrin, Photofrin) | Worldwide | - | Lung, esophagus, bile duct, bladder, brain, ovarian |
| ALA (5-aminolevulinic acid, Levulan) | Worldwide | - | Skin, bladder, brain, esophagus |
| ALA esters | Europe | - | Skin, bladder |
| Temoporfin (m-tetrahydroxy phenylchlorin, Foscan) | Europe | USA | Head, neck, lung, brain, skin, bile duct |
| Verteporfin | Europe | UK | Ophthalmic (age-related macular degeneration), pancreatic, skin |
| HPPH (2-(1-hexyloxyethyl)-2-devinyl pyropheophorbide-a) | - | USA | Head, neck, esophagus, lung |
| SnEt2 (tin ethyl etiopurpurin, Purlytin) | - | USA | Skin, breast |
| Talaporfin (mono-(l)-aspartylchlorin-e6) | USA | Liver, colon, brain | |
| Ce6-PVP (chlorin e6-polyvinypyrroidone, Fotolon), Ce6 derivatives (photodithazine, Radachlorin) | - | Belarus, Russia | Nasopharyngeal, sarcoma, brain |
| Silicon phthalocyanine | - | USA | Cutaneous T-cell lymphoma |
| Padoporfin (pallado-porphyrin, TOOKAD) | - | USA | Prostate |
| Motexafin lutetium (Lutex) | - | USA | Breast |
| Parameters | Lanthr | A1 ∙ 4 acetone |
|---|---|---|
| chemical formula | C28H16N2 | C116H112F12N4O24Ru4S4 |
| formula weight | 380.43 | 2706.61 |
| crystal system | monoclinic | triclinic |
| space group | P21 | P-1 |
| crystal size (mm3) | 0.23 × 0.13 × 0.12 | 0.25 × 0.18 × 0.14 |
| crystal color and shape | colorless rod | yellow plate |
| a (Å) | 5.0015(6) | 16.0557(11) |
| b (Å) | 16.944(2) | 20.7694(15) |
| c (Å) | 11.5374(15) | 21.3136(14) |
| α (°) | 90 | 115.639(5) |
| β (°) | 92.433(10) | 98.267(5) |
| γ (°) | 90 | 101.194(6) |
| cell volume (Å3) | 976.9(2) | 6073.6(8) |
| T (K) | 293(2) | 203(2) |
| Z | 2 | 2 |
| scan range (°) | 1.77 < θ < 29.30 | 1.48 < θ < 29.33 |
| ρcalcd (g cm−3) | 1.293 | 1.480 |
| μ (mm−1) | 0.076 | 0.644 |
| unique reflections | 5272 | 32944 |
| reflections used [I > 2σ(I)] | 1378 | 9666 |
| Rint | 0.1715 | 0.2498 |
| final R indices [I > 2σ(I) [a] | 0.0799, wR2 0.1714 | 0.0911, wR2 0.2228 |
| R indices (all data) [b] | 0.2499, wR2 0.2388 | 0.2609, wR2 0.2939 |
| GOF [c] | 0.805 | 0.836 |
| max, min Δρ/e (Å−3) | 0.268, −0.196 | 1.627, −1.213 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaschard, M.; Nehzat, F.; Cheminel, T.; Therrien, B. Arene Ruthenium Metalla-Assemblies with Anthracene Moieties for PDT Applications. Inorganics 2018, 6, 97. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics6030097
Gaschard M, Nehzat F, Cheminel T, Therrien B. Arene Ruthenium Metalla-Assemblies with Anthracene Moieties for PDT Applications. Inorganics. 2018; 6(3):97. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics6030097
Chicago/Turabian StyleGaschard, Marie, Farzaneh Nehzat, Thomas Cheminel, and Bruno Therrien. 2018. "Arene Ruthenium Metalla-Assemblies with Anthracene Moieties for PDT Applications" Inorganics 6, no. 3: 97. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics6030097