Development and Validation of Liquid Chromatography-Based Methods to Assess the Lipophilicity of Cytotoxic Platinum(IV) Complexes
Abstract
:1. Introduction
2. Results and Discussion
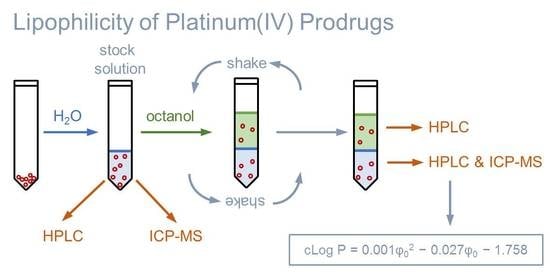
2.1. Determining Partition Coefficients by the Shake Flask Method
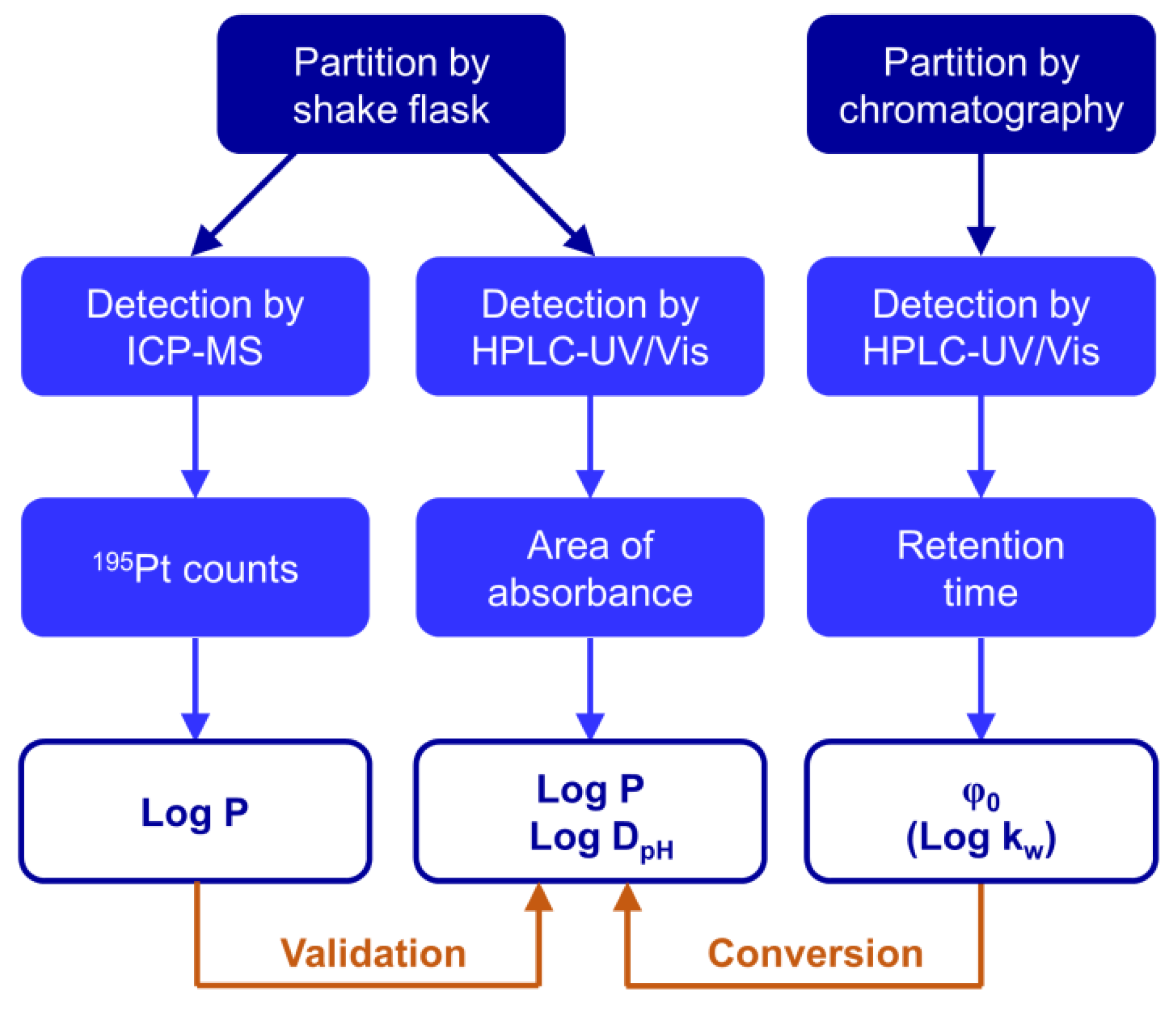
2.2. Extending the Approach to Determine Distribution Coefficients
2.3. Determination of the Chromatographic Lipophilicity Parameter φ0
2.4. Converting Chromatographic Lipophilicity to Shake Flask Lipophilicity
3. Materials and Methods
3.1. Platinum(IV) Complexes
3.2. (OC-6-43)-Amminedichlorido(cyclohexaneamine)bis[(4-ethoxy)-4-oxobutanoato]platinum(IV) (16)
3.3. (OC-6-54)-Dichlorido(N,N-dimethyl-ethane-1,2-diamine)hydroxido[4-(2-propyn-1-ylamino)-4-oxo-butanoato]platinum(IV) (27)
3.4. (OC-6-33)-Dichlorido(ethane-1,2-diamine)bis[(4-(2-propyn-1-ylamino)-4-oxobutanoato]platinum(IV) (29)
3.5. (OC-6-33)-Dichlorido(ethane-1,2-diamine)bis[(4-dimethylamino)-4-oxobutanoato]platinum(IV) (31)
3.6. Instrumentation
3.7. Shake Flask Procedure
3.8. Analysis of Samples Obtained from the Shake Flask Procedure
3.9. Determining the Distribution Coefficient by the Shake Flask Method with HPLC–UV/Vis Detection
3.10. Determining the Lipophilicity from Chromatographic Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Waring, M.J. Lipophilicity in Drug Discovery. Expert Opin. Drug Discov. 2010, 5, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.V.S.; Radi, Z.A.; Rotter, C.J.; Litchfield, J.; El-Kattan, A.F.; Lyubimov, A.V. Pharmacokinetics and Toxicokinetics in Drug Discovery and Development. In Encyclopedia of Drug Metabolism and Interactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Mälkiä, A.; Murtomäki, L.; Urtti, A.; Kontturi, K. Drug Permeation in Biomembranes: In Vitro and In Silico Prediction and Influence of Physicochemical Properties. Eur. J. Pharm. Sci. 2004, 23, 13–47. [Google Scholar] [CrossRef] [PubMed]
- Hansch, C.; Maloney, P.P.; Fujita, T. Correlation of Biological Activity of Phenoxyacetic Acids with Hammett Substituent Constants and Partition Coefficients. Nature 1962, 194, 178–180. [Google Scholar] [CrossRef]
- Hansch, C.; Fujita, T. p-s-p Analysis. A Method for the Correlation of Biological Activity and Chemical Structure. J. Am. Chem. Soc. 1964, 86, 1616–1626. [Google Scholar] [CrossRef]
- Leo, A.; Hansch, C.; Elkins, D. Partition Coefficients and Their Uses. Chem. Rev. 1971, 71, 525–616. [Google Scholar] [CrossRef]
- OECD. Test No. 107: Partition Coefficient (n-Octanol/Water): Shake Flask Method; OECD Publishing: Paris, France, 1995. [Google Scholar]
- Albert, A. Selective Toxicity: The Physicochemical Basis of Therapy; Chapman and Hall: London, UK, 1979. [Google Scholar]
- Kerns, E.H. High Throughput Physicochemical Profiling for Drug Discovery. J. Pharm. Sci. 2001, 90, 1838–1858. [Google Scholar] [CrossRef] [PubMed]
- Nasal, A.; Siluk, D.; Kaliszan, R. Chromatographic Retention Parameters in Medicinal Chemistry and Molecular Pharmacology. Curr. Med. Chem. 2003, 10, 381–426. [Google Scholar] [CrossRef] [PubMed]
- Valkó, K. Application of High-Performance Liquid Chromatography Based Measurements of Lipophilicity to Model Biological Distribution. J. Chromatogr. A 2004, 1037, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Soczewinski, E.; Wachtmeister, C.A. The Relation between the Composition of Certain Ternary Two-Phase Solvent Systems and RM Values. J. Chromatogr. A 1962, 7, 311–320. [Google Scholar] [CrossRef]
- Snyder, L.R.; Dolan, J.W.; Gant, J.R. Gradient Elution in High-Performance Liquid Chromatography: I. Theoretical Basis for Reversed-Phase Systems. J. Chromatogr. A 1979, 165, 3–30. [Google Scholar] [CrossRef]
- Valkó, K.; Slégel, P. New Chromatographic Hydrophobicity Index (φ0) Based on the Slope and the Intercept of the log k versus Organic Phase Concentration Plot. J. Chromatogr. A 1993, 631, 49–61. [Google Scholar] [CrossRef]
- Souchard, J.P.; Ha, T.T.; Cros, S.; Johnson, N.P. Hydrophobicity Parameters for Platinum Complexes. J. Med. Chem. 1991, 34, 863–864. [Google Scholar] [CrossRef] [PubMed]
- Screnci, D.; McKeage, M.J.; Galettis, P.; Hambley, T.W.; Palmer, B.D.; Baguley, B.C. Relationships Between Hydrophobicity, Reactivity, Accumulation and Peripheral Nerve Toxicity of a Series of Platinum Drugs. Br. J. Cancer 2000, 82, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Robillard, M.S.; Galanski, M.; Zimmermann, W.; Keppler, B.K.; Reedijk, J. (Aminoethanol)dichloroplatinum(II) Complexes: Influence of the Hydroxyethyl Moiety on 5′-GMP and DNA Binding, Intramolecular Stability, the Partition Coefficient and Anticancer Activity. J. Inorg. Biochem. 2002, 88, 254–259. [Google Scholar] [CrossRef]
- Hall, M.D.; Amjadi, S.; Zhang, M.; Beale, P.J.; Hambley, T.W. The Mechanism of Action of Platinum(IV) Complexes in Ovarian Cancer Cell Lines. J. Inorg. Biochem. 2004, 98, 1614–1624. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, A.; Aceto, M.; Cassino, C.; Gabano, E.; Osella, D. Uptake of Antitumor Platinum(II)-Complexes by Cancer Cells, Assayed by Inductively Coupled Plasma Mass Spectrometry (ICP-MS). J. Inorg. Biochem. 2004, 98, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Platts, J.A.; Oldfield, S.P.; Reif, M.M.; Palmucci, A.; Gabano, E.; Osella, D. The RP-HPLC Measurement and QSPR Analysis of Log Po/w Values of Several Pt(II) Complexes. J. Inorg. Biochem. 2006, 100, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Reithofer, M.R.; Bytzek, A.K.; Valiahdi, S.M.; Kowol, C.R.; Groessl, M.; Hartinger, C.G.; Jakupec, M.A.; Galanski, M.; Keppler, B.K. Tuning of Lipophilicity and Cytotoxic Potency by Structural Variation of Anticancer Platinum(IV) Complexes. J. Inorg. Biochem. 2011, 105, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D. The Mechanism of Action of Platinum Anticancer Agents—What Do We Really Know About it? Dalton Trans. 2009, 10681–10689. [Google Scholar] [CrossRef]
- Bytzek, A.K.; Reithofer, M.R.; Galanski, M.; Groessl, M.; Keppler, B.K.; Hartinger, C.G. The First Example of MEEKC-ICP-MS Coupling and its Application for the Analysis of Anticancer Platinum Complexes. Electrophoresis 2010, 31, 1144–1150. [Google Scholar] [CrossRef]
- Varbanov, H.P.; Valiahdi, S.M.; Kowol, C.R.; Jakupec, M.A.; Galanski, M.; Keppler, B.K. Novel Tetracarboxylatoplatinum(IV) Complexes as Carboplatin Prodrugs. Dalton Trans. 2012, 41, 14404–14415. [Google Scholar] [CrossRef] [PubMed]
- Ermondi, G.; Caron, G.; Ravera, M.; Gabano, E.; Bianco, S.; Platts, J.A.; Osella, D. Molecular interaction fields vs. quantum-mechanical-based descriptors in the modelling of lipophilicity of platinum(IV) complexes. Dalton Trans. 2013, 42, 3482–3489. [Google Scholar] [CrossRef] [PubMed]
- Varbanov, H.P.; Göschl, S.; Heffeter, P.; Theiner, S.; Roller, A.; Jensen, F.; Jakupec, M.A.; Berger, W.; Galanski, M.; Keppler, B.K. A Novel Class of Bis- and Tris-Chelate Diam(m)inebis(dicarboxylato)platinum(IV) Complexes as Potential Anticancer Prodrugs. J. Med. Chem. 2014, 57, 6751–6764. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, S.P.; Hall, M.D.; Platts, J.A. Calculation of Lipophilicity of a Large, Diverse Dataset of Anticancer Platinum Complexes and the Relation to Cellular Uptake. J. Med. Chem. 2007, 50, 5227–5237. [Google Scholar] [CrossRef] [PubMed]
- Tetko, I.V.; Jaroszewicz, I.; Platts, J.A.; Kuduk-Jaworska, J. Calculation of Lipophilicity for Pt(II) Complexes: Experimental Comparison of Several Methods. J. Inorg. Biochem. 2008, 102, 1424–1437. [Google Scholar] [CrossRef] [PubMed]
- Platts, J.A.; Ermondi, G.; Caron, G.; Ravera, M.; Gabano, E.; Gaviglio, L.; Pelosi, G.; Osella, D. Molecular and Statistical Modeling of Reduction Peak Potential and Lipophilicity of Platinum(IV) Complexes. J. Biol. Inorg. Chem. 2011, 16, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Tetko, I.V.; Varbanov, H.P.; Galanski, M.; Talmaciu, M.; Platts, J.A.; Ravera, M.; Gabano, E. Prediction of LogP for Pt(II) and Pt(IV) Complexes: Comparison of Statistical and Quantum-Chemistry Based Approaches. J. Inorg. Biochem. 2016, 156, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.M.; Hanif, M.; Adhireksan, Z.; Pichler, V.; Novak, M.; Jirkovsky, E.; Jakupec, M.A.; Arion, V.B.; Davey, C.A.; Keppler, B.K.; et al. Novel Metal(II) Arene 2-Pyridinecarbothioamides: A Rationale to Orally Active Organometallic Anticancer Agents. Chem. Sci. 2013, 4, 1837–1846. [Google Scholar] [CrossRef]
- Pichler, V.; Goschl, S.; Meier, S.M.; Roller, A.; Jakupec, M.A.; Galanski, M.; Keppler, B.K. Bulky (N,N)-(Di)alkylethane-1,2-diamineplatinum(II) Compounds as Precursors for Generating Unsymmetrically Substituted Platinum(IV) Complexes. Inorg. Chem. 2013, 52, 8151–8162. [Google Scholar] [CrossRef]
- Varbanov, H.P.; Jakupec, M.A.; Roller, A.; Jensen, F.; Galanski, M.; Keppler, B.K. Theoretical Investigations and Density Functional Theory Based Quantitative Structure-Activity Relationships Model for Novel Cytotoxic Platinum(IV) Complexes. J. Med. Chem. 2013, 56, 330–344. [Google Scholar] [CrossRef]
- Meier-Menches, S.M.; Gerner, C.; Berger, W.; Hartinger, C.G.; Keppler, B.K. Structure-Activity Relationships for Ruthenium and Osmium Anticancer Agents—Towards Clinical Development. Chem. Soc. Rev. 2018, 47, 909–928. [Google Scholar] [CrossRef] [PubMed]
- Varbanov, H.; Valiahdi, S.M.; Legin, A.A.; Jakupec, M.A.; Roller, A.; Galanski, M.; Keppler, B.K. Synthesis and Characterization of Novel Bis(carboxylato)dichloridobis(ethylamine)platinum(IV) Complexes with Higher Cytotoxicity than Cisplatin. Eur. J. Med. Chem. 2011, 46, 5456–5464. [Google Scholar] [CrossRef] [PubMed]
- Low, Y.W.; Blasco, F.; Vachaspati, P. Optimised Method to Estimate Octanol Water Distribution Coefficient (Log D) in a High Throughput Format. Eur. J. Pharm. Sci. 2016, 92, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.D.; Mellor, H.R.; Callaghan, R.; Hambley, T.W. Basis for Design and Development of Platinum(IV) Anticancer Complexes. J. Med. Chem. 2007, 50, 3403–3411. [Google Scholar] [CrossRef]
- Schoenmakers, P.J.; Billiet, H.A.H.; Tussen, R.; De Galan, L. Gradient Selection in Reversed-Phase Liquid Chromatography. J. Chromatogr. A 1978, 149, 519–537. [Google Scholar] [CrossRef]
- Baczek, T.; Markuszewski, M.; Kaliszan, R.; van Straten, M.A.; Claessens, H.A. Linear and Quadratic Relationships between Retention and Organic Modifier Content in Eluent in Reversed Phase High-Performance Liquid Chromatography: A Systematic Comparative Statistical Study. J. High Resolut. Chromatogr. 2000, 23, 667–676. [Google Scholar] [CrossRef]
- Goschl, S.; Varbanov, H.P.; Theiner, S.; Jakupec, M.A.; Galanski, M.; Keppler, B.K. The Role of the Equatorial Ligands for the Redox Behavior, Mode of Cellular Accumulation and Cytotoxicity of Platinum(IV) Prodrugs. J. Inorg. Biochem. 2016, 160, 264–274. [Google Scholar] [CrossRef]
- Hofer, D.; Varbanov, H.P.; Hejl, M.; Jakupec, M.A.; Roller, A.; Galanski, M.; Keppler, B.K. Impact of the Equatorial Coordination Sphere on the Rate of Reduction, Lipophilicity and Cytotoxic Activity of Platinum(IV) Complexes. J. Inorg. Biochem. 2017, 174, 119–129. [Google Scholar] [CrossRef]
- Hofer, D.; Varbanov, H.P.; Legin, A.; Jakupec, M.A.; Roller, A.; Galanski, M.; Keppler, B.K. Tetracarboxylatoplatinum(IV) Complexes Featuring Monodentate Leaving Groups—A Rational Approach Towards Exploiting the Platinum(IV) Prodrug Strategy. J. Inorg. Biochem. 2015, 153, 259–271. [Google Scholar] [CrossRef]
- Reithofer, M.; Galanski, M.; Roller, A.; Keppler, B.K. An Entry to Novel Platinum Complexes: Carboxylation of Dihydroxoplatinum(IV) Complexes with Succinic Anhydride and Subsequent Derivatization. Eur. J. Inorg. Chem. 2006, 2006, 2612–2617. [Google Scholar] [CrossRef]
- Reithofer, M.R.; Valiahdi, S.M.; Jakupec, M.A.; Arion, V.B.; Egger, A.; Galanski, M.; Keppler, B.K. Novel Di- and Tetracarboxylatoplatinum(IV) Complexes. Synthesis, Characterization, Cytotoxic Activity and DNA Platination. J. Med. Chem. 2007, 50, 6692–6699. [Google Scholar] [CrossRef] [PubMed]
- Reithofer, M.R.; Schwarzinger, A.; Valiahdi, S.M.; Galanski, M.; Jakupec, M.A.; Keppler, B.K. Novel Bis(carboxylato)dichlorido(ethane-1,2-diamine)platinum(IV) Complexes with Exceptionally High Cytotoxicity. J. Inorg. Biochem. 2008, 102, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Pichler, V.; Valiahdi, S.M.; Jakupec, M.A.; Arion, V.B.; Galanski, M.; Keppler, B.K. Mono-Carboxylated Diaminedichloridoplatinum(IV) Complexes—Selective Synthesis, Characterization and Cytotoxicity. Dalton Trans. 2011, 40, 8187–8192. [Google Scholar] [CrossRef] [PubMed]
- Pichler, V.; Heffeter, P.; Valiandi, S.M.; Kowol, C.R.; Egger, A.; Berger, W.; Jakupec, M.A.; Galanski, M.; Keppler, B.K. Unsymmetric Mono- and Dinuclear Platinum(IV) Complexes Featuring an Ethylene Glycol Moiety: Synthesis, Characterization, and Biological Activity. J. Med. Chem. 2012, 55, 11052–11061. [Google Scholar] [CrossRef] [PubMed]

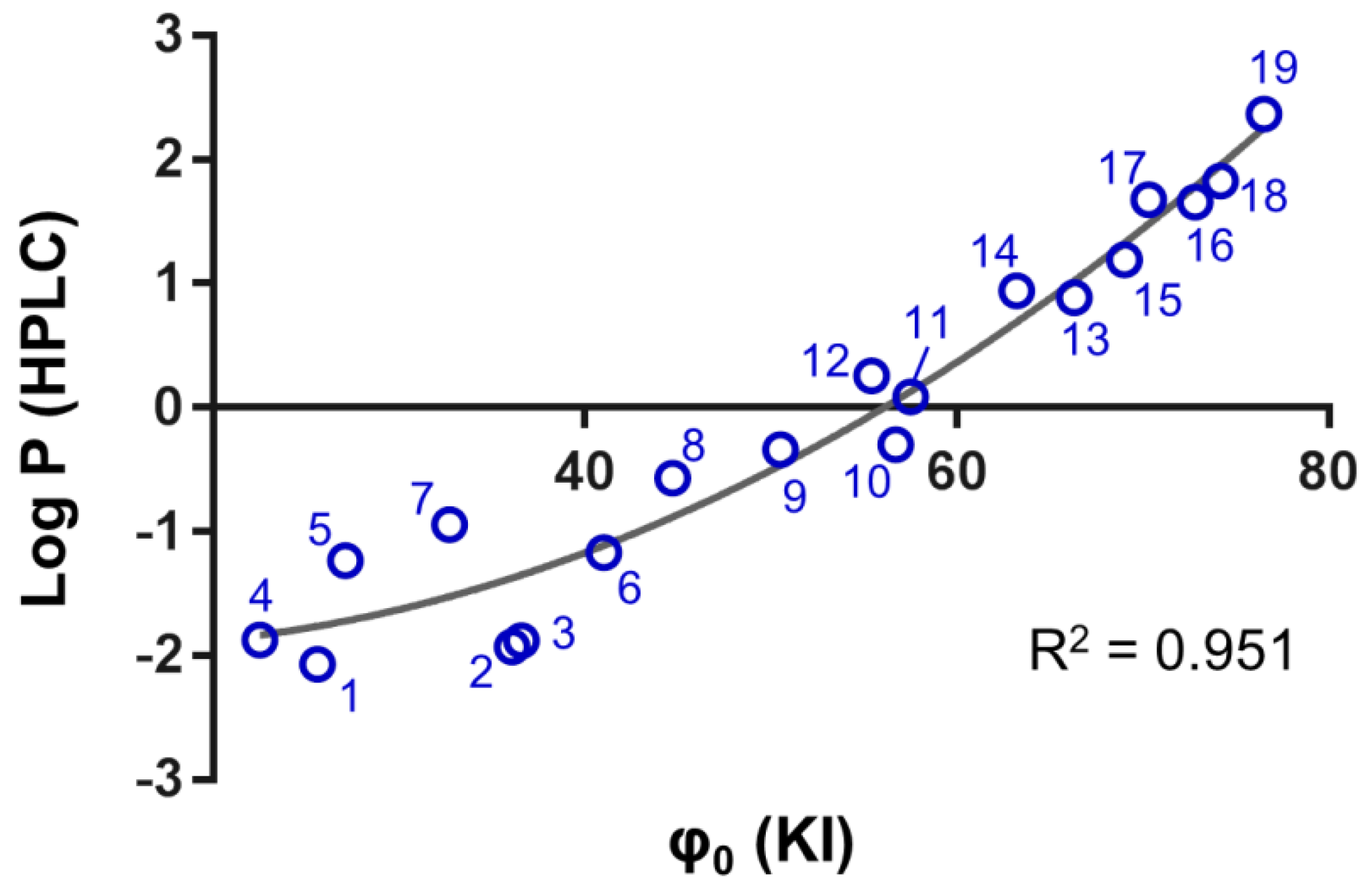
| Nr. | Structure | Log kw (KI) | φ0 (KI) | Log P (HPLC) | Log P (ICP-MS) |
|---|---|---|---|---|---|
| 1 |  | 1.14 | 25.6 | −2.07 | −1.70 |
| 2 |  | 1.60 | 36.1 | −1.93 | −2.23 |
| 3 |  | 1.47 | 36.6 | −1.88 | −1.41 |
| 4 |  | 0.76 | 22.5 | −1.87 | −1.70 |
| 5 |  | 1.07 | 27.1 | −1.23 | −1.42 |
| 6 |  | 1.39 | 41.0 | −1.17 | −1.24 |
| 7 |  | 1.18 | 32.7 | −0.94 | −1.00 |
| 8 |  | 1.77 | 44.7 | −0.57 | −0.66 |
| 9 |  | 1.86 | 50.5 | −0.34 | −0.35 |
| 10 |  | 2.28 | 56.7 | −0.30 | −0.39 |
| 11 |  | 2.67 | 57.5 | 0.09 | 0.06 |
| 12 |  | 2.20 | 55.4 | 0.26 | 0.23 |
| 13 |  | 3.14 | 66.3 | 0.89 | 0.77 |
| 14 |  | 3.03 | 63.2 | 0.94 | 0.71 |
| 15 |  | 3.26 | 69.0 | 1.19 | 0.95 |
| 16 |  | 4.04 | 72.8 | 1.66 | 1.20 |
| 17 |  | 3.48 | 70.3 | 1.68 | 1.30 |
| 18 |  | 3.86 | 74.2 | 1.83 | 1.33 |
| 19 |  | 4.18 | 76.5 | 2.37 | 1.06 |
| Complex | pH | |||||||
|---|---|---|---|---|---|---|---|---|
| 1.7 | 2.5 | 3.7 | 4.2 | 4.7 | 5.6 | 6.2 | 7.4 | |
| 20 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 21 | −1.56 | −1.54 | −1.82 | −2.16 | −2.91 | n.d. | n.d. | n.d. |
| 22 | −1.19 | −1.26 | −1.42 | −1.69 | −2.03 | n.d. | n.d. | n.d. |
| 23 | −0.35 | −0.39 | −0.56 | −0.91 | −1.36 | −2.73 | n.d. | n.d. |
| 24 | 0.46 | 0.45 | 0.37 | 0.15 | −0.23 | −1.34 | −2.17 | n.d. |
| 10 | −0.25 | −0.28 | −0.24 | −0.27 | −0.27 | −0.27 | −0.29 | −0.27 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klose, M.H.M.; Theiner, S.; Varbanov, H.P.; Hoefer, D.; Pichler, V.; Galanski, M.S.; Meier-Menches, S.M.; Keppler, B.K. Development and Validation of Liquid Chromatography-Based Methods to Assess the Lipophilicity of Cytotoxic Platinum(IV) Complexes. Inorganics 2018, 6, 130. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics6040130
Klose MHM, Theiner S, Varbanov HP, Hoefer D, Pichler V, Galanski MS, Meier-Menches SM, Keppler BK. Development and Validation of Liquid Chromatography-Based Methods to Assess the Lipophilicity of Cytotoxic Platinum(IV) Complexes. Inorganics. 2018; 6(4):130. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics6040130
Chicago/Turabian StyleKlose, Matthias H. M., Sarah Theiner, Hristo P. Varbanov, Doris Hoefer, Verena Pichler, Mathea Sophia Galanski, Samuel M. Meier-Menches, and Bernhard K. Keppler. 2018. "Development and Validation of Liquid Chromatography-Based Methods to Assess the Lipophilicity of Cytotoxic Platinum(IV) Complexes" Inorganics 6, no. 4: 130. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics6040130