Imidazo-Phenanthroline Ligands as a Convenient Modular Platform for the Preparation of Heteroleptic Cu(I) Photosensitizers
Abstract
:1. Introduction
2. Results and Discussion
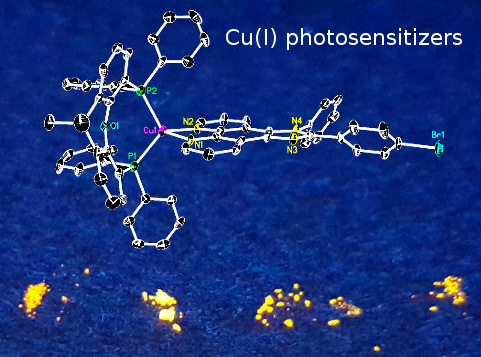
2.1. Synthesis and Structural Characterization
2.2. Electrochemical and Photophysical Studies
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smalley, R.E. Future Global Energy Prosperity: The Terawatt Challenge. MRS Bull. 2005, 30, 412–417. [Google Scholar] [CrossRef] [Green Version]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [Green Version]
- Armaroli, N.; Balzani, V. The Future of Energy Supply: Challenges and Opportunities. Angew. Chem. Int. Ed. 2007, 46, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Schiermeier, Q.; Tollefson, J.; Scully, T.; Witze, A.; Morton, O. Electricity without Carbon. Nature 2008, 454, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Styring, S. Artificial photosynthesis for solar fuels. Faraday Discuss. 2012, 155, 357–376. [Google Scholar] [CrossRef] [PubMed]
- Hammarström, L. Catalyst: Chemistry’s Role in Providing Clean and Affordable Energy for All. Chem 2016, 1, 515–518. [Google Scholar] [CrossRef]
- Service, R.F. Is It Time to Shoot for the Sun? Science 2005, 309, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Armaroli, N.; Balzani, V. Solar Electricity and Solar Fuels: Status and Perspectives in the Context of the Energy Transition. Chem. Eur. J. 2016, 22, 32–57. [Google Scholar] [CrossRef]
- Armaroli, N. Photoactive mono- and polynuclear Cu(I)-phenanthrolines. A viable alternative to Ru(II)-polypyridines? Chem. Soc. Rev. 2001, 30, 113–124. [Google Scholar] [CrossRef]
- Eckenhoff, W.T.; Eisenberg, R. Molecular systems for light driven hydrogen production. Dalton Trans. 2012, 41, 13004–13021. [Google Scholar] [CrossRef]
- Frischmann, P.D.; Mahata, K.; Würthner, F. Powering the future of molecular artificial photosynthesis with light-harvesting metallosupramolecular dye assemblies. Chem. Soc. Rev. 2013, 42, 1847–1870. [Google Scholar] [CrossRef]
- Yuan, Y.-J.; Yu, Z.-T.; Chen, D.-Q.; Zou, Z.-G. Metal-complex chromophores for solar hydrogen generation. Chem. Soc. Rev. 2017, 46, 603–631. [Google Scholar] [CrossRef]
- Costa, R.D.; Ortí, E.; Bolink, H.J.; Monti, F.; Accorsi, G.; Armaroli, N. Luminescent Ionic Transition-Metal Complexes for Light-Emitting Electrochemical Cells. Angew. Chem. Int. Ed. 2012, 51, 8178–8211. [Google Scholar] [CrossRef]
- Zhang, Y.; Schulz, M.; Wächtler, M.; Karnahl, M.; Dietzek, B. Heteroleptic diamine-diphosphine Cu(I) complexes as an alternative towards noble-metal based photosensitizers: Design strategies, photophysical properties and perspective applications. Coord. Chem. Rev. 2018, 356, 127–146. [Google Scholar] [CrossRef]
- Lazorski, M.S.; Castellano, F.N. Advances in the light conversion properties of Cu(I)-based photosensitizers. Polyhedron 2014, 82, 57–70. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Constable, E.C. The emergence of copper(I)-based dye sensitized solar cells. Chem. Soc. Rev. 2015, 44, 8386–8398. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances in organic light-emitting devices comprising copper complexes: A realistic approach for low-cost and highly emissive devices? Org. Electron. 2015, 21, 27–39. [Google Scholar] [CrossRef]
- Weber, M.D.; Fresta, E.; Elie, M.; Miehlich, M.E.; Renaud, J.-L.; Meyer, K.; Gaillard, S.; Costa, R.D. Rationalizing Fabrication and Design Toward Highly Efficient and Stable Blue Light-Emitting Electrochemical Cells Based on NHC Copper(I) Complexes. Adv. Funct. Mater. 2018, 28, 1707423. [Google Scholar] [CrossRef]
- Armaroli, N.; Accorsi, G.; Cardinali, F.; Cardinali, F. Photochemistry and Photophysics of Coordination Compounds: Copper. Top. Curr. Chem. 2007, 69–115. [Google Scholar] [CrossRef]
- Tschierlei, S.; Karnahl, M.; Rockstroh, N.; Junge, H.; Beller, M.; Lochbrunner, S. Substitution-Controlled Excited State Processes in Heteroleptic Copper(I) Photosensitizers Used in Hydrogen Evolving Systems. ChemPhysChem 2014, 15, 3709–3713. [Google Scholar] [CrossRef]
- Iwamura, M.; Takeuchi, S.; Tahara, T. Ultrafast Excited-State Dynamics of Copper(I) Complexes. Acc. Chem. Res. 2015, 48, 782–791. [Google Scholar] [CrossRef]
- Mara, M.W.; Fransted, K.A.; Chen, L.X. Interplays of excited state structures and dynamics in copper(I) diimine complexes: Implications and perspectives. Coord. Chem. Rev. 2015, 282–283, 2–18. [Google Scholar] [CrossRef]
- Paria, S.; Reiser, O. Copper in Photocatalysis. ChemCatChem 2014, 6, 2477–2483. [Google Scholar] [CrossRef]
- Hernandez-Perez, A.C.; Collins, S.K. Heteroleptic Cu-Based Sensitizers in Photoredox Catalysis. Acc. Chem. Res. 2016, 49, 1557–1565. [Google Scholar] [CrossRef]
- Luo, S.-P.; Mejía, E.; Friedrich, A.; Pazidis, A.; Junge, H.; Surkus, A.-E.; Jackstell, R.; Denurra, S.; Gladiali, S.; Lochbrunner, S.; Beller, M. Photocatalytic Water Reduction with Copper-Based Photosensitizers: A Noble-Metal-Free System. Angew. Chem. 2013, 125, 437–441. [Google Scholar] [CrossRef]
- Mejía, E.; Luo, S.-P.; Karnahl, M.; Friedrich, A.; Tschierlei, S.; Surkus, A.-E.; Junge, H.; Gladiali, S.; Lochbrunner, S.; Beller, M. A Noble-Metal-Free System for Photocatalytic Hydrogen Production from Water. Chem. Eur. J. 2013, 19, 15972–15978. [Google Scholar] [CrossRef]
- Balzani, V.; Juris, A.; Barigelletti, F.; Campagna, S.; Belser, P.; Von Zelewsky, A. Ru(II)-Polypyridine-complexes: Photophysics, Photochemistry, Electrochemistry and Chemiluminescence. Coord. Chem. Rev. 1988, 84, 85–277. [Google Scholar] [CrossRef]
- Schäfer, B.; Görls, H.; Meyer, S.; Henry, W.; Vos, J.G.; Rau, S. Synthesis and Properties of Tetrasubstituted 1,10-Phenanthrolines and Their Ruthenium Complexes. Eur. J. Inorg. Chem 2007, 4056–4063. [Google Scholar] [CrossRef]
- Troian-Gautier, L.; Moucheron, C. RutheniumII Complexes bearing Fused Polycyclic Ligands: From Fundamental Aspects to Potential Applications. Molecules 2014, 19, 5028–5087. [Google Scholar] [CrossRef]
- Navarro, M.; Cisneros-Fajardo, E.J.; Sierralta, A.; Fernández-Mestre, M.; Silva, P.; Arrieche, D.; Marchán, E. Design of copper DNA intercalators with leishmanicidal activity. J. Biol. Inorg. Chem. 2003, 8, 401–408. [Google Scholar] [CrossRef]
- Sandroni, M.; Maufroy, A.; Rebarz, M.; Pellegrin, Y.; Blart, E.; Ruckebusch, C.; Poizat, O.; Sliwa, M.; Odobel, F. Design of Efficient Photoinduced Charge Separation in Donor-Copper(I)-Acceptor Triad. J. Phys. Chem. C 2014, 118, 28388–28400. [Google Scholar] [CrossRef]
- Heberle, M.; Tschierlei, S.; Rockstroh, N.; Ringenberg, M.; Frey, W.; Junge, H.; Beller, M.; Lochbrunner, S.; Karnahl, M. Heteroleptic Copper Photosensitizers: Why an Extended π-System Does Not Automatically Lead to Enhanced Hydrogen Production. Chem. Eur. J. 2017, 23, 312–319. [Google Scholar] [CrossRef]
- Zhang, Y.; Traber, P.; Zedler, L.; Kupfer, S.; Gräfe, S.; Schulz, M.; Frey, W.; Karnahl, M.; Dietzek, B. Cu(I) vs. Ru(II) photosensitizers: Elucidation of electron transfer processes within a series of structurally related complexes containing an extended π-system. Phys. Chem. Chem. Phys. 2018, 20, 24843–24857. [Google Scholar] [CrossRef]
- Soulis, K.; Gourlaouen, C.; Daniel, C.; Quatela, A.; Odobel, F.; Blart, E.; Pellegrin, Y. New luminescent copper(I) complexes with extended π-conjugation. Polyhedron 2018, 140, 42–50. [Google Scholar] [CrossRef]
- Jin, C.; Liu, J.; Chen, Y.; Zeng, L.; Guan, R.; Ouyang, C.; Ji, L.; Chao, H. Cyclometalated Iridium(III) Complexes as Two-Photon Phosphorescent Probes for Specific Mitochondrial Dynamics Tracking in Living Cells. Chem. Eur. J. 2015, 21, 12000–12010. [Google Scholar] [CrossRef]
- Jadhav, T.; Choi, J.M.; Lee, J.Y.; Dhokale, B.; Misra, R. Non-doped blue organic light emitting devices based on tetraphenylethylene-π-imidazole derivatives. Org. Electron. 2016, 37, 448–452. [Google Scholar] [CrossRef]
- Kuang, S.-M.; Cuttell, D.G.; McMillin, D.R.; Fanwick, P.E.; Walton, R.A. Synthesis and Structural Characterization of Cu(I) and Ni(II) Complexes that Contain the Bis[2-(diphenylphosphino)phenyl]ether Ligand. Novel Emission Properties for the Cu(I) Species. Inorg. Chem. 2002, 41, 3313–3322. [Google Scholar] [CrossRef]
- Fischer, S.; Hollmann, D.; Tschierlei, S.; Karnahl, M.; Rockstroh, N.; Barsch, E.; Schwarzbach, P.; Luo, S.-P.; Junge, H.; Beller, M.; et al. Death and Rebirth: Photocatalytic Hydrogen Production by a Self-Organizing Copper-Iron System. ACS Catal. 2014, 4, 1845–1849. [Google Scholar] [CrossRef]
- Zhang, Y.; Heberle, M.; Wächtler, M.; Karnahl, M.; Dietzek, B. Determination of side products in the photocatalytic generation of hydrogen with copper photosensitizers by resonance Raman spectroelectrochemistry. RSC Adv. 2016, 6, 105801–105805. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.-Z.; Ye, B.-H.; Wang, L.; Ji, L.-N.; Zhou, J.-Y.; Li, R.-H.; Zhou, Z.-Y. Bis(2,2’-bipyridine)ruthenium(II) complexes with imidazo[4,5-f][1,10]-phenanthroline or 2-phenylimidazo[4,5-f][1,10]phenanthroline. J. Chem. Soc. Dalton Trans. 1997, 1395–1402. [Google Scholar] [CrossRef]
- Peuntinger, K.; Pilz, T.D.; Staehle, R.; Schaub, M.; Kaufhold, S.; Petermann, L.; Wunderlin, M.; Görls, H.; Heinemann, F.W.; Li, J.; et al. Carbene based photochemical molecular assemblies for solar driven hydrogen generation. Dalton Trans. 2014, 43, 13683–13695. [Google Scholar] [CrossRef] [Green Version]
- Petermann, L.; Staehle, R.; Pfeifer, M.; Reichardt, C.; Sorsche, D.; Wächtler, M.; Popp, J.; Dietzek, B.; Rau, S. Oxygen-Dependent Photocatalytic Water Reduction with a Ruthenium(imidazolium) Chromophore and a Cobaloxime Catalyst. Chem. Eur. J. 2016, 22, 8240–8253. [Google Scholar] [CrossRef]
- Hedley, G.J.; Ruseckas, A.; Samuel, I.D.W. Vibrational Energy Flow Controls Internal Conversion in a Transition Metal Complex. J. Phys. Chem. A 2010, 114, 8961–8968. [Google Scholar] [CrossRef]
- Lennox, A.J.J.; Fischer, S.; Jurrat, M.; Luo, S.-P.; Rockstroh, N.; Junge, H.; Ludwig, R.; Beller, M. Copper-Based Photosensitisers in Water Reduction: A More Efficient In Situ Formed System and Improved Mechanistic Understanding. Chem. Eur. J. 2016, 22, 1233–1238. [Google Scholar] [CrossRef]
- Shi, L.; Li, B. A Series of CuI Complexes Containing 1,10-Phenanthroline Derivative Ligands: Synthesis, Characterization, Photophysical, and Oxygen-Sensing Properties. Eur. J. Inorg. Chem. 2009, 2009, 2294–2302. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef]
- Bruker, S. APEX2 and SAINT; Bruker AXs Inc.: Madison, WI, USA, 2008. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Wilfred, L.A.; Christina, L.L.C. Purification of Laboratory Chemicals, 6th ed.; Butterworth–Heinemann: Oxford, UK, 2009; ISBN 978-1-85617-567-8. [Google Scholar]







| C1 | C2 | R1 a | |
|---|---|---|---|
| Cu–N1 [pm] | 206.6(7) | 209.0(4) | 208.4(3) |
| Cu–N2 [pm] | 201.6(9) | 204.6(4) | 211.2(3) |
| Cu–P1 [pm] | 222.9(2) | 224.14(17) | 226.26(11) |
| Cu–P2 [pm] | 227.5(3) | 227.23(16) | 228.63(13) |
| N1–Cu–N2 [°] | 79.7(3) | 80.48(17) | 80.53(13) |
| P1–Cu–P2 [°] | 118.75(8) | 113.21(6) | 112.93(4) |
| N1–Cu–P1 [°] | 101.8(2) | 104.81(12) | 108.77(9) |
| N1–Cu–P2 [°] | 127.1(2) | 118.88(12) | 120.33(9) |
| N2–Cu–P1 [°] | 115.3(2) | 125.26(13) | 120.33(9) |
| N2–Cu–P2 [°] | 106.8(2) | 110.45(12) | 118.59(9) |
| Comp. | λmax,abs [nm] (ε [103 M−1 cm−1]) | λmax,em [nm] ACN | λmax,em [nm] (LC) DCM | λmax,em [nm] (MLCT) DCM | λmax,em [nm] solid | τ [ns] solid | E1/2 red [V] | E ox1 [V] a | E ox2 [V] a |
|---|---|---|---|---|---|---|---|---|---|
| R1 | 378 (3.1) b | 564 b | 518 c | 167, 1702 c | −2.10 b | 0.82 b | - | ||
| C1 | 403 (6.00) | 474 | 454 | 618 | 573 | 14, 286 | −2.01 | 0.73 | 0.99 |
| C2 | 400 (5.73) | 457 | 452 | 613 | 574 | 12, 426 | −1.98 | 0.85 | 1.11 |
| C3 | 403 (8.64) | 474 | 454 | 614 | 564 | 15, 438 | −1.97 | 0.55 | 0.65 |
| C4 | 401 (6.49) | 478 | 454 | 611 | 576 | 20, 438 | −1.97 | 0.77 | 1.02 |
| L1 | 274 (59.05) | 408 | - | 1.19 | 1.52 | ||||
| L2 | 277 (64.77) | 409 | - | 1.25 | 1.46 | ||||
| L3 | 283 (41.11) | 413 | - | 1.23 | 1.46 | ||||
| L4 | 284 (40.02) | 410 | - | 1.22 | 1.43 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmid, M.-A.; Rentschler, M.; Frey, W.; Tschierlei, S.; Karnahl, M. Imidazo-Phenanthroline Ligands as a Convenient Modular Platform for the Preparation of Heteroleptic Cu(I) Photosensitizers. Inorganics 2018, 6, 134. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics6040134
Schmid M-A, Rentschler M, Frey W, Tschierlei S, Karnahl M. Imidazo-Phenanthroline Ligands as a Convenient Modular Platform for the Preparation of Heteroleptic Cu(I) Photosensitizers. Inorganics. 2018; 6(4):134. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics6040134
Chicago/Turabian StyleSchmid, Marie-Ann, Martin Rentschler, Wolfgang Frey, Stefanie Tschierlei, and Michael Karnahl. 2018. "Imidazo-Phenanthroline Ligands as a Convenient Modular Platform for the Preparation of Heteroleptic Cu(I) Photosensitizers" Inorganics 6, no. 4: 134. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics6040134