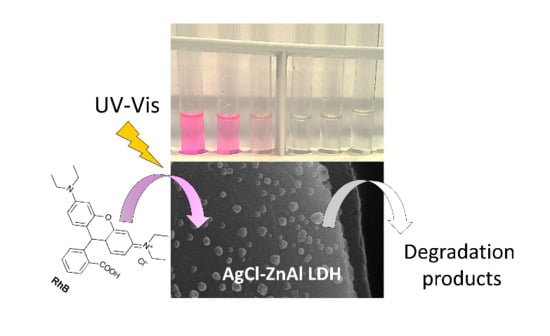
AgCl-ZnAl Layered Double Hydroxides as Catalysts with Enhanced Photodegradation and Antibacterial Activities
Abstract
:1. Introduction
2. Results and Discussion
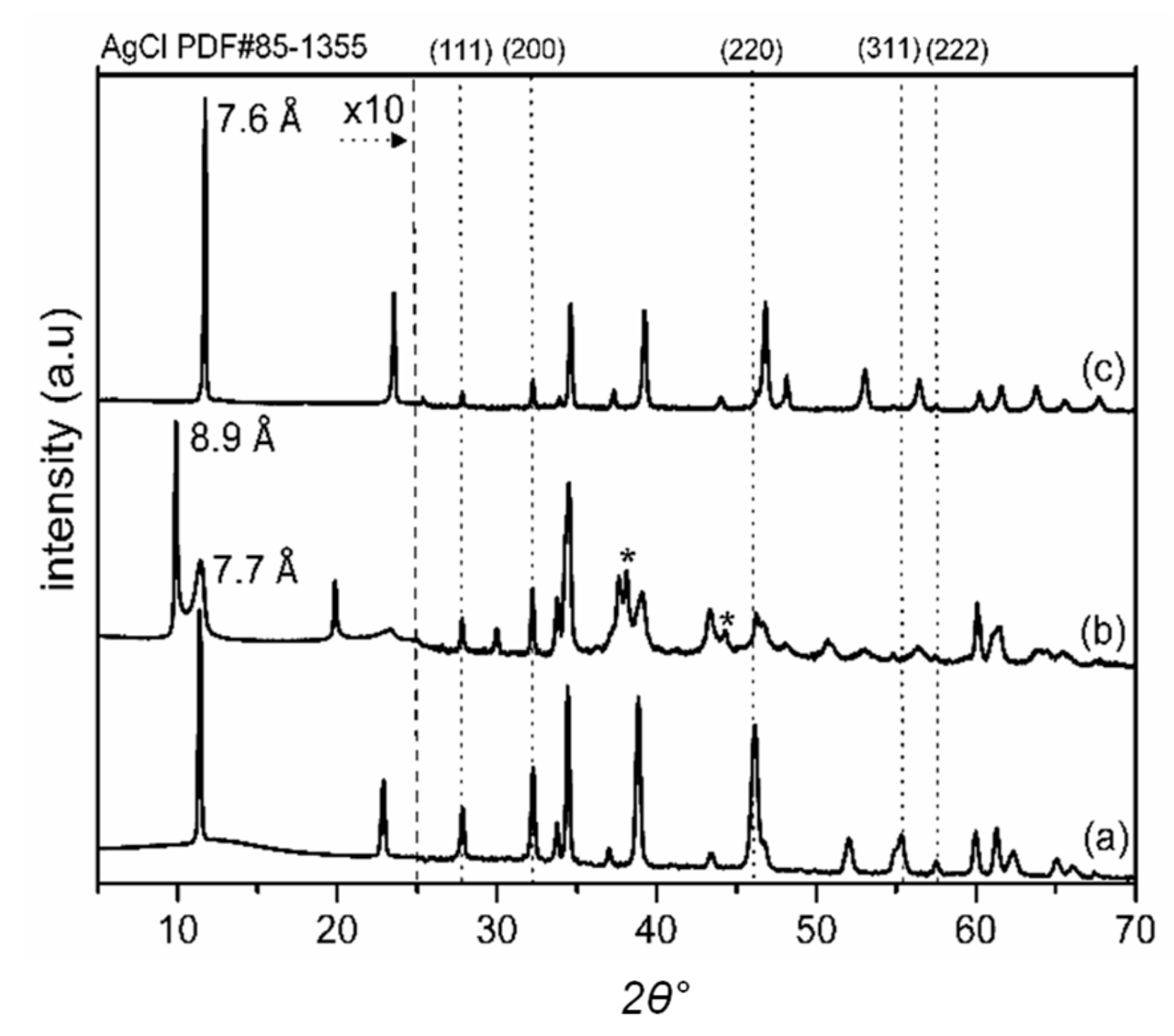
2.1. Characterization of Catalysts
2.2. Photodegradation Experiments
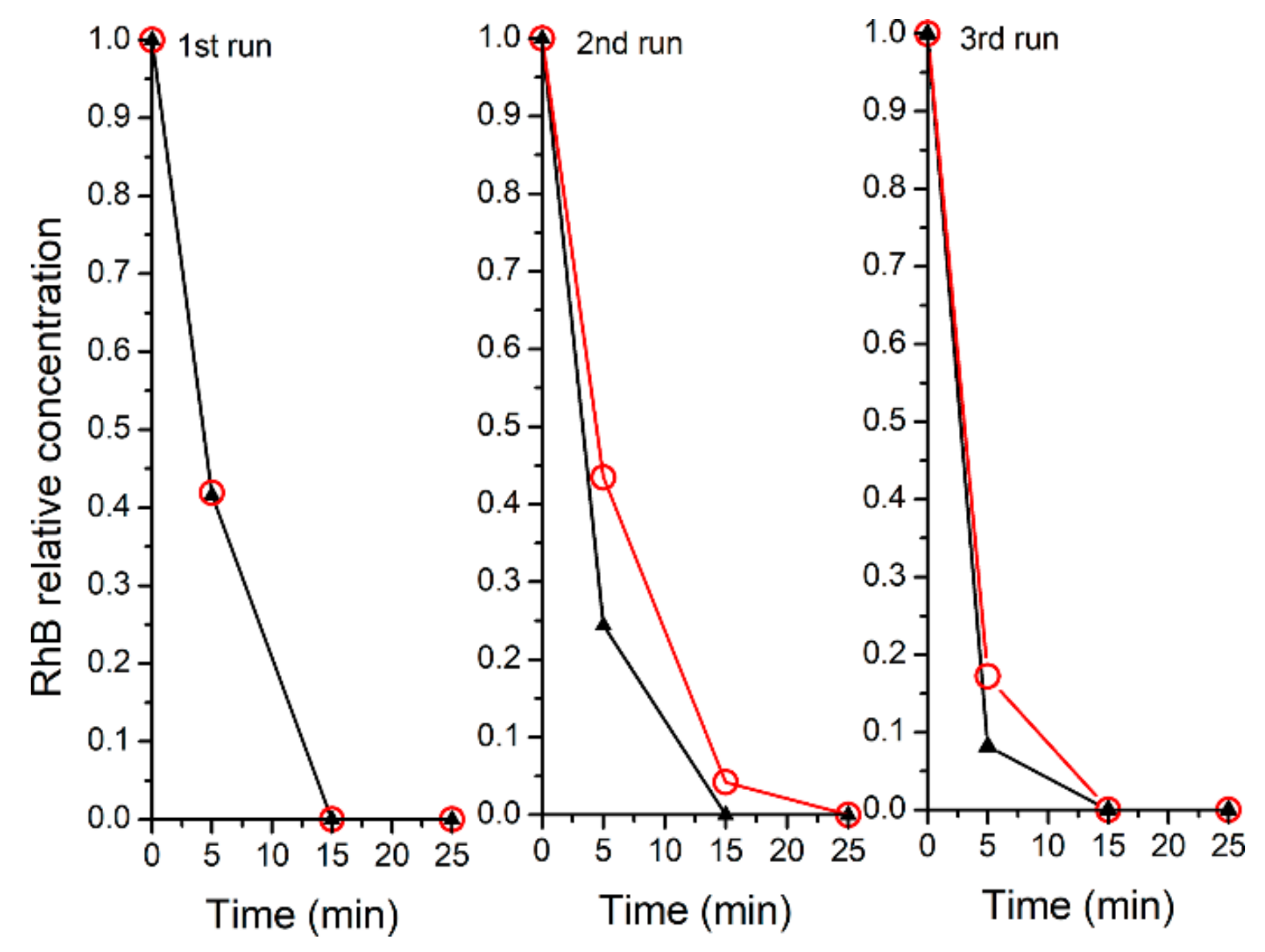
2.3. Recyclability of the Catalysts
2.4. Mechanism of Photocatalytic Degradation
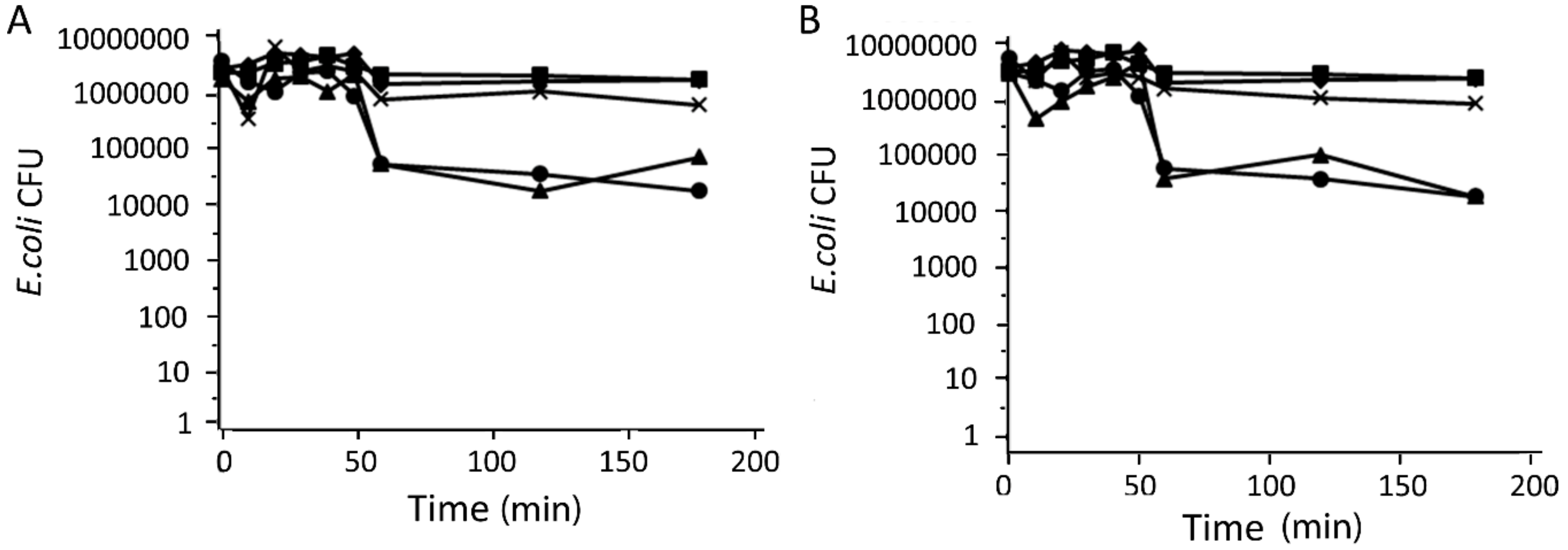
2.5. Antibacterial Activity of Catalysts
3. Materials and Methods
3.1. Chemicals
3.2. Catalysts Synthesis
3.3. Instrumental Procedures
3.4. Photodegradation Experiments
3.5. Antimicrobial Performance Test
3.6. Time-Kill Curve Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Litter, M.I.; Candal, R.J.; Meichtry, J.M. (Eds.) Advanced Oxidation Technologies—Sustainable Solutions for Environmental Treatments, 1st ed.; CRC Press/Balkema: Leiden, The Netherlands, 2014; Volume 9. [Google Scholar]
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A Review of Removal of Pollutants from Water/Wastewater Using Different Types of Nanomaterials. Adv. Mater. Sci. Eng. 2014, 2014, 825910. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Chang, T.F.M.; Chen, C.Y.; Sone, M.; Hsu, Y.J. Mechanistic Insights into Photodegradation of Organic Dyes Using Heterostructure Photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef]
- Wu, M.J.; Wu, J.Z.; Zhang, J.; Chen, H.; Zhou, J.Z.; Qian, G.R.; Xu, Z.P.; Du, Z.; Rao, Q.L. A review on fabricating heterostructures from layered double hydroxides for enhanced photocatalytic activities. Catal. Sci. Technol. 2018, 8, 1207–1228. [Google Scholar] [CrossRef]
- Lin, W.-H.; Chiu, Y.-H.; Shao, P.-W.; Hsu, Y.-J. Metal-Particle-Decorated ZnO Nanocrystals: Photocatalysis and Charge Dynamics. ACS Appl. Mater. Interfaces 2016, 8, 32754–32763. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Liu, T.-C.; Hsu, Y.-J. ZnSe·0.5N2H4 Hybrid Nanostructures: A Promising Alternative Photocatalyst for Solar Conversion. ACS Appl. Mater. Interfaces 2015, 7, 1616–1623. [Google Scholar] [CrossRef]
- Pu, Y.-C.; Chou, H.-Y.; Kuo, W.-S.; We, K.-H.; Hsu, Y.J. Interfacial charge carrier dynamics of cuprous oxide-reduced graphene oxide (Cu2O-rGO) nanoheterostructures and their related visible-light-driven photocatalysis. Appl. Catal. B Environ. 2017, 204, 21–32. [Google Scholar] [CrossRef]
- Pu, Y.-C.; Lin, W.-H.; Hsu, Y.-J. Modulation of charge carrier dynamics of NaxH2−xTi3O7–Au–Cu2O Z-scheme nanoheterostructures through size effect. Appl. Catal. B Environ. 2015, 163, 343–351. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Lin, W.-H.; Lu, Y.-H.; Chiou, Y.-D.; Hsu, Y.-J. First demonstration of rainbow photocatalysts using ternaryCd1−xZnxSe nanorods of varying compositions. Appl. Catal. A General. 2014, 476, 140–147. [Google Scholar] [CrossRef]
- Chen, M.-Y.; Hsu, Y.-J. Type-II nanorod heterostructure formation through one-step cation exchange. Nanoscale 2013, 5, 363–368. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Hsu, Y.-J. Interfacial charge carrier dynamics of type-II semiconductor nanoheterostructures. Appl. Catal. B Environ. 2013, 130, 93–98. [Google Scholar] [CrossRef]
- Guo, J.-L.; Chiou, Y.-D.; Liang, W.-I.; Liu, H.-J.; Chen, Y.-J.; Kuo, W.-C.; Tsai, C.-Y.; Tsai, K.-A.; Kuo, H.-H.; Hsieh, W.-F.; et al. Complex Oxide–Noble Metal Conjugated Nanoparticles. Adv. Mater. 2013, 25, 2040–2044. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Pu, Y.-C.; Hsu, Y.-J. Interfacial Charge Carrier Dynamics of the Three-Component In2O3–TiO2–Pt Heterojunction System. J. Phys. Chem. C 2012, 116, 2967–2975. [Google Scholar] [CrossRef]
- Chen, W.-T.; Hsu, Y.-J. L-Cysteine-Assisted Growth of Core-Satellite ZnS-Au Nanoassemblies with High Photocatalytic Efficiency. Langmuir 2010, 26, 5918–5925. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.-C.; Chen, Y.-C.; Hsu, Y.-J. Au-decorated NaxH2−xTi3O7 nanobelts exhibiting remarkable photocatalytic properties under visible-light illumination. Appl. Catal. B Environ. 2010, 97, 389–397. [Google Scholar] [CrossRef]
- Keane, D.A.; McGuigan, K.G.; Ibáñez, P.F.; Polo-López, M.I.; Byrne, J.A.; Dunlop, P.S.M.; O’Shea, K.; Dionysiou, D.D.; Pillai, C.S. Solar photocatalysis for water disinfection: Materials and reactor design. Catal. Sci. Technol. 2014, 4, 1211–1226. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Piccirillo, C.; Pullar, R.C.; Gualtieri, A.F.; Seabra, M.P.; Castro, P.M.L.; Labrincha, J.A. Silver-Modified Nano-titania as an Antibacterial Agent and Photocatalyst. J. Phys. Chem. C 2014, 118, 4751–4766. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Dai, Y.; Whangbo, M.-H. Plasmonic photocatalysts: Harvesting visible light with noble metal nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 9813–9825. [Google Scholar] [CrossRef] [PubMed]
- Pica, M.; Nocchetti, M.; Ridolfi, B.; Donnadio, A.; Costantino, F.; Gentili, P.L.; Casciola, M. Nanosized zirconium phosphate/AgCl composite materials: A new synergy for efficient photocatalytic degradation of organic dye pollutants. J. Mater. Chem. A 2015, 3, 5525–5534. [Google Scholar] [CrossRef]
- Pica, M.; Calzuola, S.; Donnadio, A.; Gentili, P.L.; Nocchetti, M.; Casciola, M. De-Ethylation and Cleavage of Rhodamine B by a Zirconium Phosphate/Silver Bromide Composite Photocatalyst. Catalysts 2019, 9, 3. [Google Scholar] [CrossRef]
- Rives, V. (Ed.) Layered Double Hydroxides: Present and Future; Nova Science Publishers: New York, NY, USA, 2001. [Google Scholar]
- Fan, H.; Zhu, J.; Sun, J.; Zhang, S.; Ai, S. Ag/AgBr/Co–Ni–NO3 Layered Double Hydroxide Nanocomposites with Highly Adsorptive and Photocatalytic Properties. Chem. Eur. J. 2013, 19, 2523–2530. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Y.; Cheng, J.; Fan, H.; Zhu, J.; Wang, X.; Ai, S. Synthesis of Ag/AgCl/Zn–Cr LDHs composite with enhanced visible-light photocatalytic performance. J. Mol. Catal. A Chem. 2014, 382, 146–153. [Google Scholar] [CrossRef]
- Nocchetti, M.; Donnadio, A.; Ambrogi, V.; Andreani, P.; Bastianini, M.; Pietrella, D.; Latterini, L. Ag/AgCl nanoparticle decorated layered double hydroxides: Synthesis, characterization and antimicrobial properties. J. Mater. Chem. B 2013, 1, 2383–2393. [Google Scholar] [CrossRef]
- Bastianini, M.; Costenaro, D.; Bisio, C.; Marchese, L.; Costantino, U.; Vivani, R.; Nocchetti, M. On the Intercalation of the Iodine–Iodide Couple on Layered Double Hydroxides with Different Particle Sizes. Inorg. Chem. 2012, 51, 2560–2568. [Google Scholar] [CrossRef] [PubMed]
- Madusanka, N.; Sandaruwan, C.; Kottegoda, N.; Karunaratne, V. Synthesis of Ag Nanoparticle/Mg–Al-Layered Double Hydroxide Nanohybrids. Eur. Int. J. Appl. Sci. Technol. 2014, 1, 1–7. [Google Scholar]
- Ambrogi, V.; Donnadio, A.; Pietrella, D.; Latterini, L.; Alunni Proietti, F.; Marmottini, F.; Padeletti, G.; Kaciulis, S.; Giovagnoli, S.; Ricci, M. Chitosan films containing mesoporous SBA-15 supported silver nanoparticles for wound dressing. J. Mater. Chem. B 2014, 2, 6054–6063. [Google Scholar] [CrossRef]
- Mock, J.J.; Barbic, M.; Smith, D.R.; Schultz, D.A.; Schultz, S. Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J. Chem. Phys. 2002, 116, 6755–6759. [Google Scholar] [CrossRef]
- Watanabe, T.; Takizawa, T.; Honda, K. Photocatalysis through excitation of adsorbates. 1. Highly efficient N-deethylation of rhodamine B adsorbed to cadmium sulfide. J. Phys. Chem. 1977, 81, 1845–1851. [Google Scholar] [CrossRef]
- Inoue, T.; Watanabe, T.; Fujishima, A.; Honda, K.; Kohayakawa, K. Suppression of Surface Dissolution of CdS Photoanode by Reducing Agents. J. Electrochem. Soc. 1977, 124, 719–722. [Google Scholar] [CrossRef]
- Wu, T.; Liu, G.; Zhao, J.; Hidaka, H.; Serpone, N. Photoassisted Degradation of Dye Pollutants. V. Self-Photosensitized Oxidative Transformation of Rhodamine B under Visible Light Irradiation in Aqueous TiO2 Dispersions. J. Phys. Chem. B 1998, 102, 5845–5851. [Google Scholar] [CrossRef]
- Yang, T.T.; Chen, W.T.; Hsu, Y.J.; Wei, K.H.; Lin, T.Y.; Lin, T.W. Interfacial Charge Carrier Dynamics in Core-Shell Au-CdS Nanocrystals. J. Phys. Chem. C 2010, 114, 11414–11420. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, J.; Hidaka, H. Highly selective deethylation of rhodamine B: Adsorption and photooxidation pathways of the dye on the TiO2/SiO2 composite photocatalyst. Int. J. Photoenergy 2003, 5, 209–217. [Google Scholar] [CrossRef]
- Chen, D.; Yoo, S.H.; Huang, Q.; Ali, G.; Cho, S.O. Sonochemical Synthesis of Ag/AgCl Nanocubes and Their Efficient Visible-Light-Driven Photocatalytic Performance. Chem. Eur. J. 2012, 18, 5192–5200. [Google Scholar] [CrossRef]
- Zhuang, J.; Dai, W.; Tian, Q.; Li, Z.; Xie, L.; Wang, J.; Liu, P. Photocatalytic Degradation of RhB over TiO2 Bilayer Films: Effect of Defects and Their Location. Langmuir 2010, 26, 9686–9694. [Google Scholar] [CrossRef]
- Yu, K.; Yang, S.; He, H.; Sun, C.; Gu, C.; Ju, Y. Visible Light-Driven Photocatalytic Degradation of Rhodamine B over NaBiO3: Pathways and Mechanism. J. Phys. Chem. A 2009, 113, 10024–10032. [Google Scholar] [CrossRef]
- Xu, H.; Li, H.; Xia, J.; Yin, S.; Luo, Z.; Liu, L.; Xu, L. One-Pot Synthesis of Visible-Light-Driven Plasmonic Photocatalyst Ag/AgCl in Ionic Liquid. ACS Appl. Mater. Interfaces 2011, 3, 22–29. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, W.; Li, J.; Zhao, J.; Hidaka, H.; Serpone, N. Formation and identification of intermediates in the visible-light-assisted photodegradation of sulforhodamine-B dye in aqueous TiO2 dispersion. J. Environ. Sci. Technol. 2002, 36, 3604–3611. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Ma, W.; Cheng, M.; Zhao, J. Efficient H2O2 Oxidation of Organic Pollutants Catalyzed by Supported Iron Sulfophenylporphyrin under Visible Light Irradiation. J. Phys. Chem. B 2004, 108, 7263–7270. [Google Scholar] [CrossRef]
- Fu, H.; Pan, C.; Yao, W.; Zhu, Y. Visible-Light-Induced Degradation of Rhodamine B by Nanosized Bi2WO6. J. Phys. Chem. B 2005, 109, 22432–22439. [Google Scholar] [CrossRef]
- Wang, P.; Cheng, M.; Zhang, Z. On different photodecomposition behaviors of rhodamine B on laponite and montmorillonite clay under visible light irradiation. J. Saudi Chem. Soc. 2014, 18, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Li, J.; Wang, X.; Huang, Y.; Zeng, M.; Xu, J. In Situ Ion Exchange Synthesis of Strongly Coupled Ag@AgCl/g-C3N4 Porous Nanosheets as Plasmonic Photocatalyst for Highly Efficient Visible-Light Photocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 22116–22125. [Google Scholar] [CrossRef]
- Xu, S.-M.; Pan, T.; Dou, Y.-B.; Yan, H.; Zhang, S.-T.; Ning, F.-Y.; Shi, W.-Y.; Wei, M. Theoretical and Experimental Study on MIIMIII-Layered Double Hydroxides as Efficient Photocatalysts toward Oxygen Evolution from Water. J. Phys. Chem. C 2015, 119, 18823–18834. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.B.; Ingram, D. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y.; Wei, J.; Whangbo, M.H. Ag@AgCl: A Highly Efficient and Stable Photocatalyst Active under Visible Light. Angew. Chem. Int. Ed. 2008, 47, 7931–7933. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, X.; Quan, X.; Chen, S.; Yu, H. Graphene Sheets Grafted Ag@AgCl Hybrid with Enhanced Plasmonic Photocatalytic Activity under Visible Light. Environ. Sci. Technol. 2011, 45, 5731–5736. [Google Scholar] [CrossRef]
- Glaus, S.; Calzaferri, G. The band structures of the silver halides AgF, AgCl, and AgBr: A comparative study. Photochem. Photobiol. Sci. 2003, 2, 398–401. [Google Scholar] [CrossRef]
- Zhou, Z.; Long, M.; Cai, W.; Cai, J. Synthesis and photocatalytic performance of the efficient visible light photocatalyst Ag–AgCl/BiVO4. J. Mol. Catal. A Chem. 2012, 353, 22–28. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- He, W.; Kim, H.K.; Wamer, W.G.; Melka, D.; Callahan, J.H.; Yin, J.J. Photogenerated Charge Carriers and Reactive Oxygen Species in ZnO/Au Hybrid Nanostructures with Enhanced Photocatalytic and Antibacterial Activity. J. Am. Chem. Soc. 2014, 136, 750–757. [Google Scholar] [CrossRef]
- Mao, C.; Xiang, Y.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wang, X.; Chu, P.K.; Wu, S. Photo-Inspired Antibacterial Activity and Wound Healing Acceleration by Hydrogel Embedded with Ag/Ag@AgCl/ZnO Nanostructures. ACS Nano 2017, 11, 9010–9021. [Google Scholar] [CrossRef]
- Costantino, U.; Marmottini, F.; Nocchetti, M.; Vivani, R. New Synthetic Routes to Hydrotalcite-Like Compounds—Characterization and Properties of the Obtained Materials. Eur. J. Inorg. Chem. 1998, 10, 1439–1446. [Google Scholar] [CrossRef]

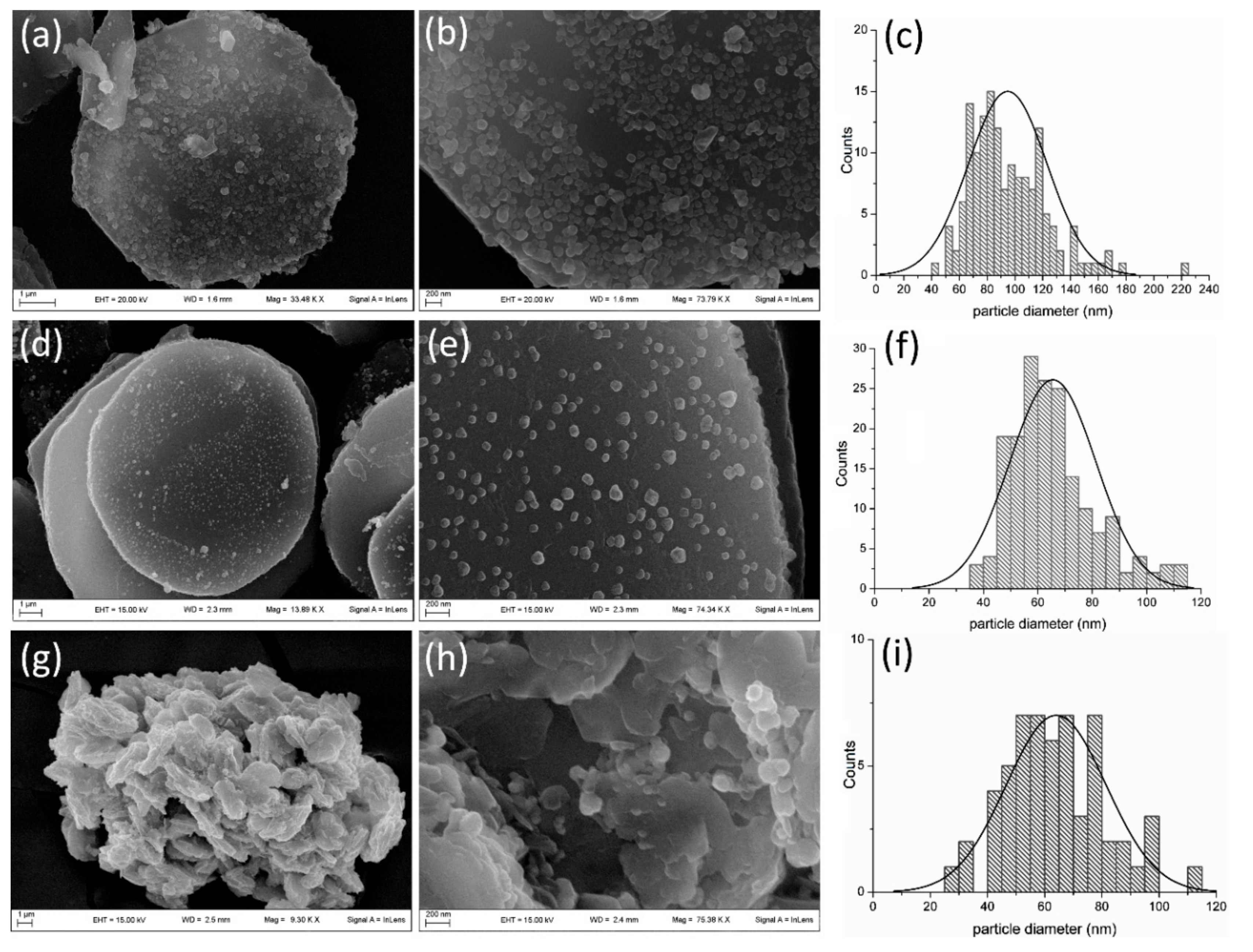





| Sample | Ag (w/w %) | 2θ (°) | FWHM (°) | AgCl Diameter (nm) | |
|---|---|---|---|---|---|
| Calculated 1 | Measured 2 ± SD | ||||
| LDH1 | 12.1 | 27.812 | 0.138 | 95.2 | 94.8 ± 28 |
| 32.224 | 0.131 | 104.4 | |||
| LDH2 | 6.3 | 27.793 | 0.162 | 79.2 | 65.5 ± 16 |
| 32.202 | 0.161 | 75.2 | |||
| LDH3 | 4.9 | 27.753 | 0.261 | 39.2 | 64.0 ± 18 |
| 32.160 | 0.224 | 48.1 | |||
| Sample | Ag (w/w %) | mg Sample/mL RhB | mg Ag/mL RhB | RhB t1/2 (min) |
|---|---|---|---|---|
| AgCl | 75.3 | 0.2 | 0.15 | 22 |
| LDH1 | 12.1 | 1.24 | 0.15 | 12 |
| LDH1 | 12.1 | 2.48 | 0.30 | <5 |
| LDH2 | 6.3 | 2.38 | 0.15 | 9 |
| LDH3 | 4.9 | 3.06 | 0.15 | <5 |
| Run | % RhB | % De-Ethylated | % Cleavage | % Cleav/% De-eth | ||||
|---|---|---|---|---|---|---|---|---|
| LDH1 | LDH3 | LDH1 | LDH3 | LDH1 | LDH3 | LDH1 | LDH3 | |
| I | 41.9 | 41.6 | 38.8 | 22.5 | 19.3 | 35.9 | 0.50 | 1.6 |
| II | 43.5 | 24.4 | 31.9 | 17.8 | 24.6 | 57.8 | 0.77 | 3.2 |
| III | 17.2 | 8.2 | 54.3 | 25.0 | 28.5 | 66.8 | 0.52 | 2.7 |
| Sample | MIC90 (μg·ml−1) |
|---|---|
| LDH1 | 9.45 |
| LDH3 | 3.83 |
| LDH | >10000 |
| Gentamicin | <0.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nocchetti, M.; Pica, M.; Ridolfi, B.; Donnadio, A.; Boccalon, E.; Zampini, G.; Pietrella, D.; Casciola, M. AgCl-ZnAl Layered Double Hydroxides as Catalysts with Enhanced Photodegradation and Antibacterial Activities. Inorganics 2019, 7, 120. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7100120
Nocchetti M, Pica M, Ridolfi B, Donnadio A, Boccalon E, Zampini G, Pietrella D, Casciola M. AgCl-ZnAl Layered Double Hydroxides as Catalysts with Enhanced Photodegradation and Antibacterial Activities. Inorganics. 2019; 7(10):120. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7100120
Chicago/Turabian StyleNocchetti, Morena, Monica Pica, Berardo Ridolfi, Anna Donnadio, Elisa Boccalon, Giulia Zampini, Donatella Pietrella, and Mario Casciola. 2019. "AgCl-ZnAl Layered Double Hydroxides as Catalysts with Enhanced Photodegradation and Antibacterial Activities" Inorganics 7, no. 10: 120. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7100120