Highlights on Gold TADF Complexes
Abstract
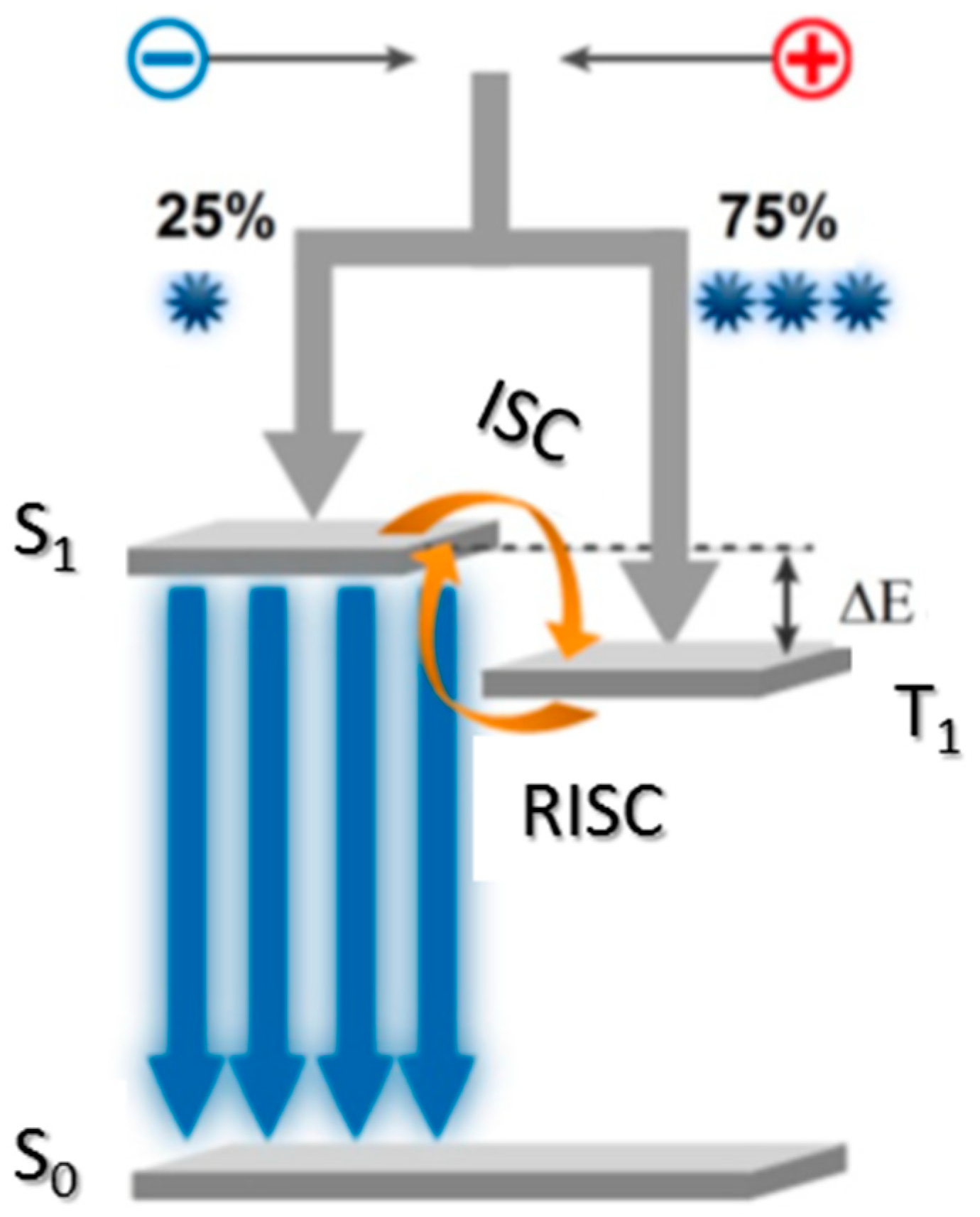
:1. Introduction
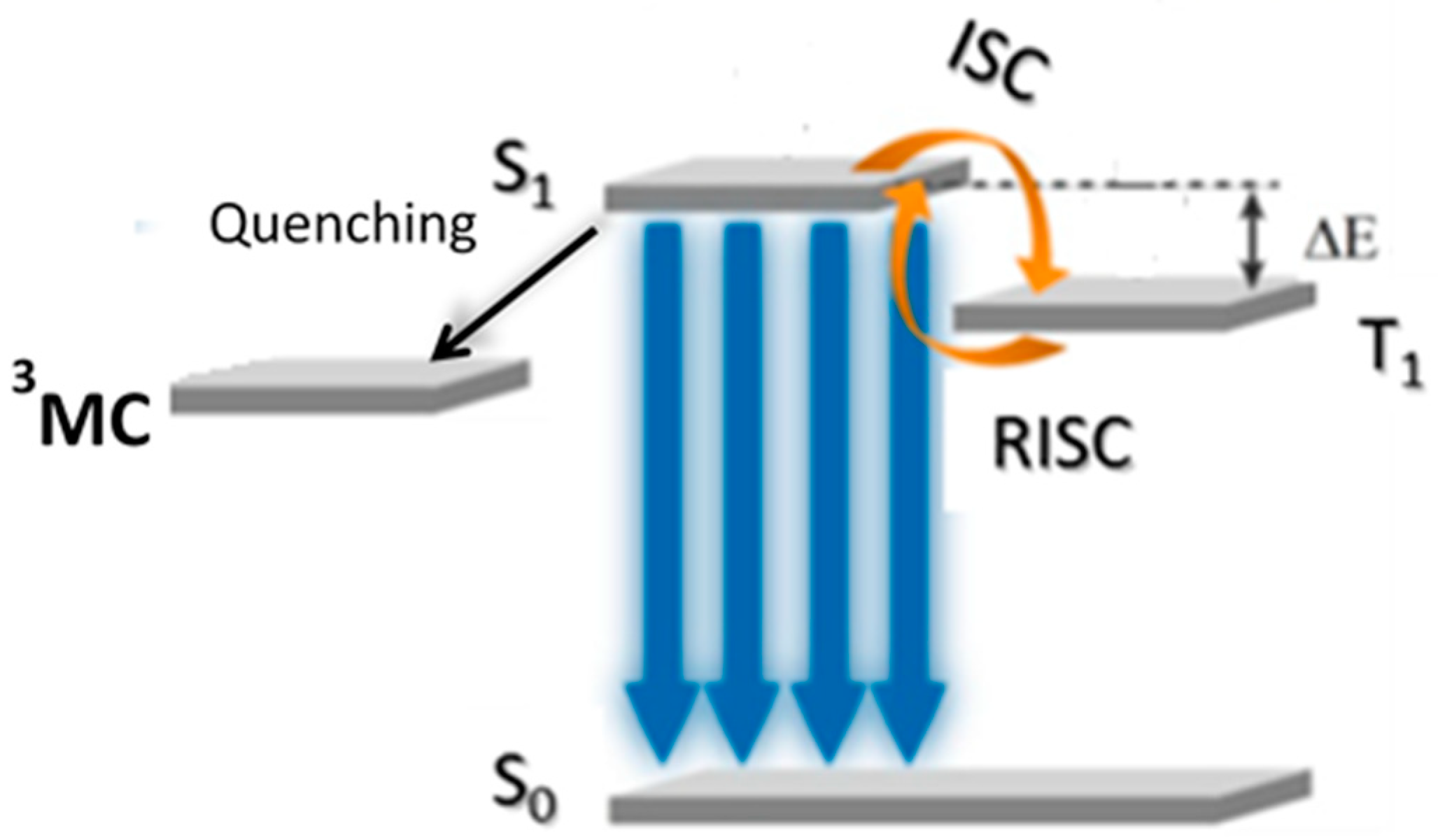
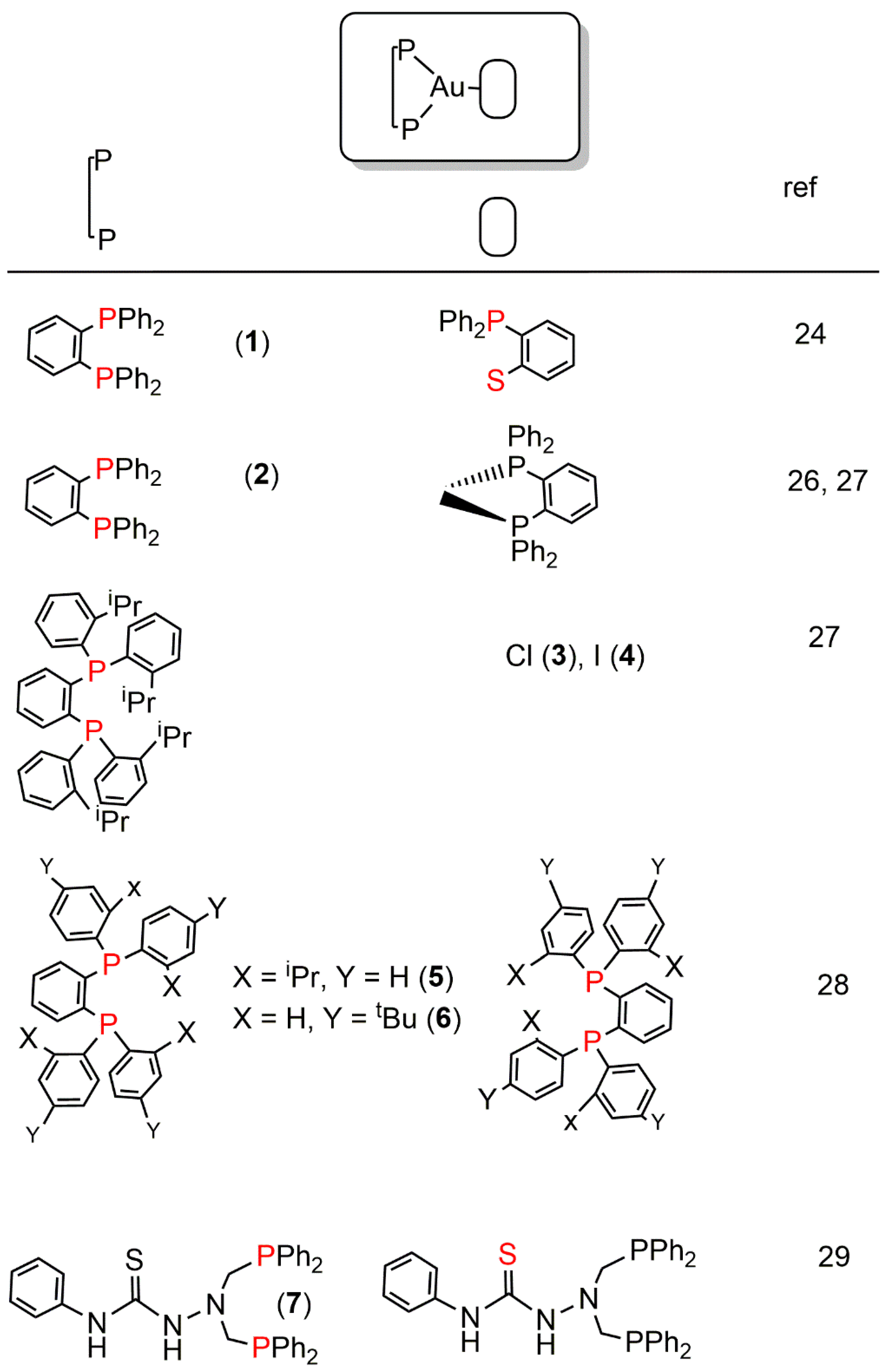
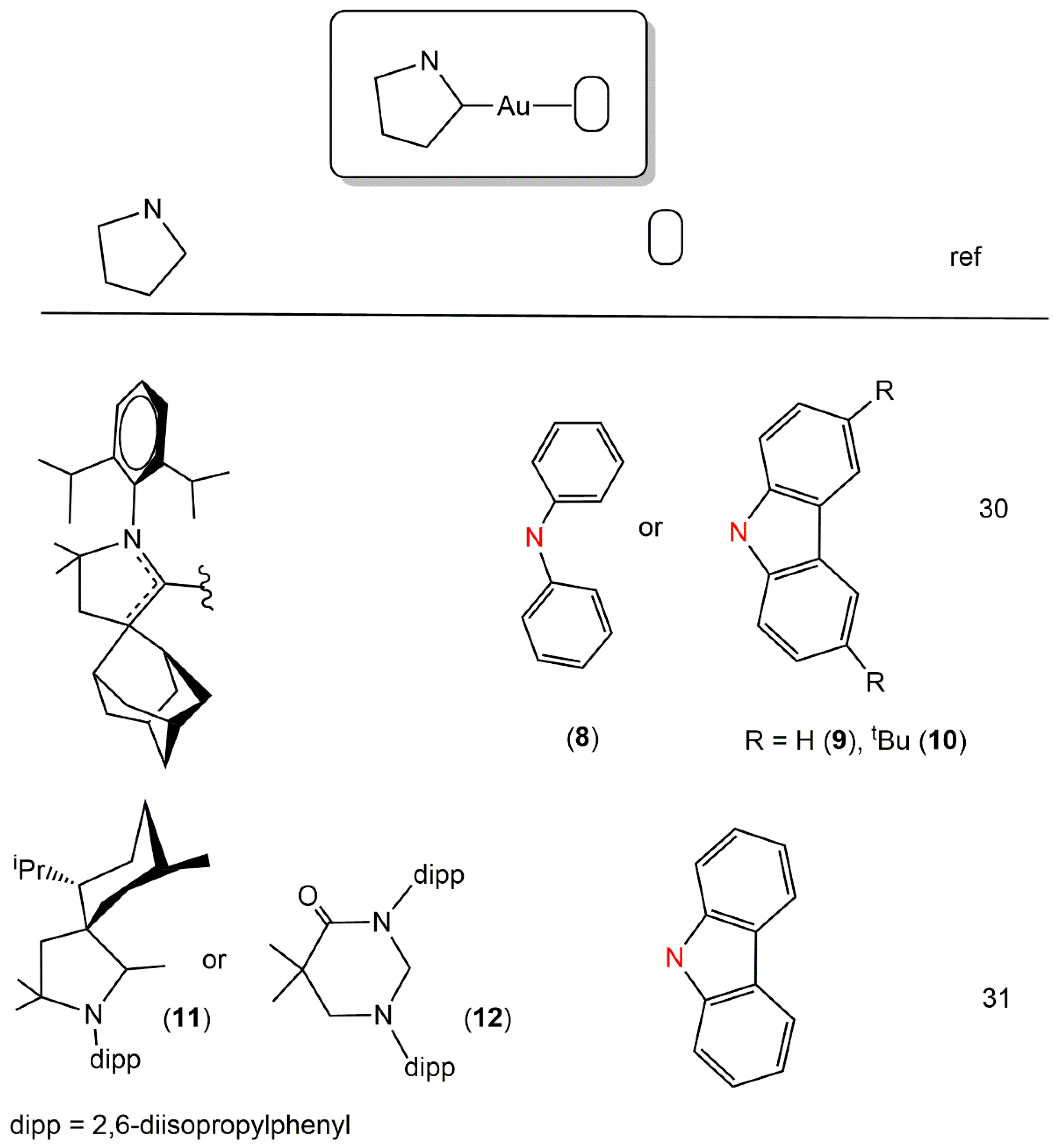
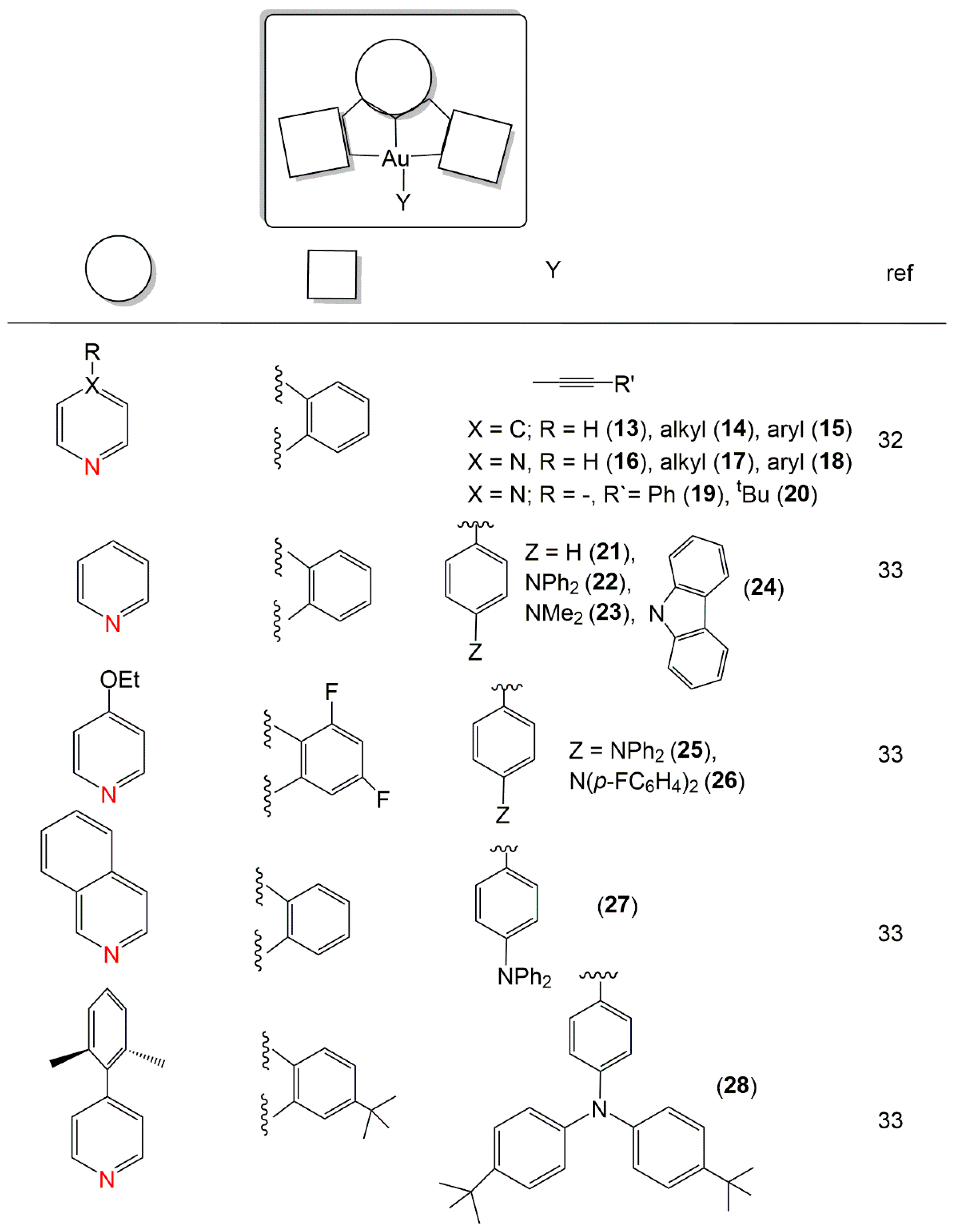
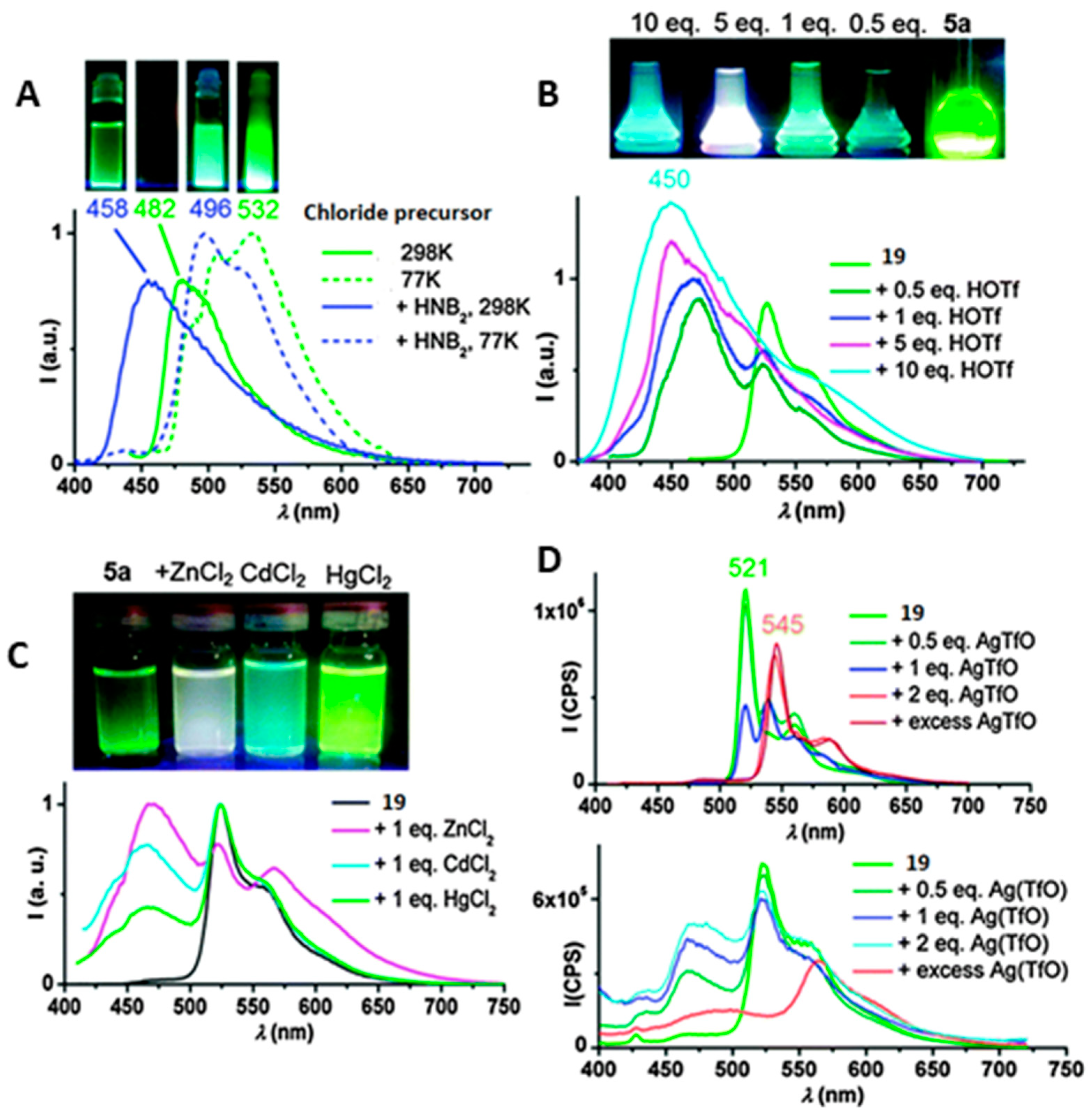
2. Au(I) Complexes
3. Au(III) Complexes
4. Conclusions and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Pashaei, B.; Karimi, S.; Shahroosvand, H.; Abbasi, P.; Pilkington, M.; Bartolotta, A.; Fresta, E.; Fernandez-Cestau, J.; Costa, R.D.; Bonaccorso, F. Polypyridyl ligands as a versatile platform for solid-state light-emitting devices. Chem. Soc. Rev. 2019, 48, 5033–5139. [Google Scholar] [CrossRef]
- Cai, M.; Zhang, D.; Duan, L. High Performance Thermally Activated Delayed Fluorescence Sensitized Organic Light-Emitting Diodes. Chem. Rec. 2019, 19, 1611–1623. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, S. Phosphorescent Pt(II) Emitters for OLEDs: From Triarylboron-Functionalized Bidentate Complexes to Compounds with Macrocyclic Chelating Ligands. Chem. Rec. 2019, 19, 1693–1709. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Shen, J.; Guo, H.; Cheng, X.; Dong, Q.; Yang, J.; Wong, W. Recent Advances in the Optimization of Organic Light-Emitting Diodes with Metal-Containing Nanomaterials. Chem. Rec. 2019, 19, 1753–1767. [Google Scholar] [CrossRef] [PubMed]
- Zampetti, A.; Minotto, A.; Cacialli, F. Near-Infrared (NIR) Organic Light-Emitting Diodes (OLEDs): Challenges and Opportunities. Adv. Funct. Mater. 2019, 29, 1807623. [Google Scholar] [CrossRef]
- Zhang, Q.C.; Xiao, H.; Zhang, X.; Xu, L.J.; Chen, Z.N. Luminescent oligonuclear metal complexes and the use in organic light-emitting diodes. Coord. Chem. Rev. 2019, 378, 121–133. [Google Scholar] [CrossRef]
- Bergmann, L.; Zink, D.M.; Bräse, S.; Baumann, T.; Volz, D. Metal–Organic and Organic TADF-Materials: Status, Challenges and Characterization. Top. Curr. Chem. 2016, 374, 22. [Google Scholar] [CrossRef]
- Wei, Q.; Ge, Z.; Voit, B. Thermally Activated Delayed Fluorescent Polymers: Structures, Properties, and Applications in OLED Devices. Macromol. Rapid Commun. 2019, 40, 1800570. [Google Scholar] [CrossRef]
- Lee, J.H.; Chen, C.H.; Lee, P.H.; Lin, H.Y.; Leung, M.; Chiu, T.L.; Lin, C.F. Blue organic light-emitting diodes: Current status, challenges, and future outlook. J. Mater. Chem. C 2019, 7, 5874–5888. [Google Scholar] [CrossRef]
- Zou, Y.; Gong, S.; Xie, G.; Yang, C. Design Strategy for Solution-Processable Thermally Activated Delayed Fluorescence Emitters and Their Applications in Organic Light-Emitting Diodes. Adv. Opt. Mater. 2018, 6, 1800568. [Google Scholar] [CrossRef]
- Jankus, V.; Data, P.; Graves, D.; McGuinness, C.; Santos, J.; Bryce, M.R.; Dias, F.B.; Monkman, A.P. Highly Efficient TADF OLEDs: How the Emitter-Host Interaction Controls Both the Excited State Species and Electrical Properties of the Devices to Achieve Near 100% Triplet Harvesting and High Efficiency. Adv. Funct. Mater. 2014, 24, 6178–6186. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Jiang, W.; Duan, L. Recent progress in solution processable TADF materials for organic light-emitting diodes. J. Mater. Chem. C 2018, 6, 5577–5596. [Google Scholar] [CrossRef]
- Zhang, Q.; Tsang, D.; Kuwabara, H.; Hatae, Y.; Li, B.; Takahashi, T.; Lee, S.Y.; Yasuda, T.; Adachi, C. Nearly 100% internal quantum efficiency in undoped electroluminescent devices employing pure organic emitters. Adv. Mater. 2015, 27, 2096–2100. [Google Scholar] [CrossRef] [PubMed]
- Volz, D.; Chen, Y.; Wallesch, M.; Liu, R.; Fléchon, C.; Zink, D.M.; Friedrichs, J.; Flügge, H.; Steininger, R.; Göttlicher, J.; et al. Bridging the Efficiency Gap: Fully Bridged Dinuclear Cu(I)-Complexes for Singlet Harvesting in High-Efficiency OLEDs. Adv. Mater. 2015, 27, 2538–2543. [Google Scholar] [CrossRef] [PubMed]
- Deaton, J.C.; Switalski, S.C.; Kondakov, D.Y.; Young, R.H.; Pawlik, T.D.; Giesen, D.J.; Harkins, S.B.; Miller, A.J.M.; Mickenberg, S.F.; Peters, J.C. E-Type Delayed Fluorescence of a Phosphine-Supported Cu2(μ-NAr2)2 Diamond Core: Harvesting Singlet and Triplet Excitons in OLEDs. J. Am. Chem. Soc. 2010, 132, 9499–9508. [Google Scholar] [CrossRef] [PubMed]
- Blasse, G.; Mcmillin, D.R. On the luminescence of bis (triphenylphosphine) phenanthroline copper(I). Chem. Phys. Lett. 1980, 70, 1–3. [Google Scholar] [CrossRef]
- Berberan-Santos, M.N.; Garcia, J.M.M. Unusually Strong Delayed Fluorescence of C 70. J. Am. Chem. Soc. 1996, 118, 9391–9394. [Google Scholar] [CrossRef]
- Endo, A.; Ogasawara, M.; Takahashi, A.; Yokoyama, D.; Kato, Y.; Adachi, C. Thermally Activated Delayed Fluorescence from Sn4+-Porphyrin Complexes and Their Application to Organic Light Emitting Diodes—A Novel Mechanism for Electroluminescence. Adv. Mater. 2009, 21, 4802–4806. [Google Scholar] [CrossRef]
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 2012, 492, 234–238. [Google Scholar] [CrossRef]
- Tao, Y.; Yuan, K.; Chen, T.; Xu, P.; Li, H.; Chen, R.; Zheng, C.; Zhang, L.; Huang, W. Thermally Activated Delayed Fluorescence Materials towards the Breakthrough of Organoelectronics. Adv. Mater. 2014, 26, 7931–7958. [Google Scholar] [CrossRef]
- Gao, Y.J.; Chen, W.K.; Zhang, T.T.; Fang, W.H.; Cui, G. Theoretical Studies on Excited-State Properties of Au(III) Emitters with Thermally Activated Delayed Fluorescence. J. Phys. Chem. C 2018, 122, 27608–27619. [Google Scholar] [CrossRef]
- To, W.P.; Chan, K.T.; Tong, G.S.M.; Ma, C.; Kwok, W.M.; Guan, X.; Low, K.H.; Che, C.M. Strongly Luminescent Gold(III) Complexes with Long-Lived Excited States: High Emission Quantum Yields, Energy Up-Conversion, and Nonlinear Optical Properties. Angew. Chem. Int. Ed. 2013, 52, 6648–6652. [Google Scholar] [CrossRef] [PubMed]
- Belyaev, A.; Eskelinen, T.; Dau, T.M.; Ershova, Y.Y.; Tunik, S.P.; Melnikov, A.S.; Hirva, P.; Koshevoy, I.O. Cyanide-Assembled d10 Coordination Polymers and Cycles: Excited State Metallophilic Modulation of Solid-State Luminescence. Chem. A Eur. J. 2018, 24, 1404–1415. [Google Scholar] [CrossRef] [PubMed]
- Osawa, M.; Kawata, I.; Ishii, R.; Igawa, S.; Hashimoto, M.; Hoshino, M. Application of neutral d10 coinage metal complexes with an anionic bidentate ligand in delayed fluorescence-type organic light-emitting diodes. J. Mater. Chem. C 2013, 1, 4375–4383. [Google Scholar] [CrossRef]
- Bizzarri, C.; Hundemer, F.; Busch, J.; Bräse, S. Triplet emitters versus TADF emitters in OLEDs: A comparative study. Polyhedron 2018, 140, 51–66. [Google Scholar] [CrossRef]
- Osawa, M.; Kawata, I.; Igawa, S.; Hoshino, M.; Fukunaga, T.; Hashizume, D. Vapochromic and Mechanochromic Tetrahedral Gold(I) Complexes Based on the 1,2-Bis(diphenylphosphino)benzene Ligand. Chem. A Eur. J. 2010, 16, 12114–12126. [Google Scholar] [CrossRef] [PubMed]
- Osawa, M.; Aino, M.A.; Nagakura, T.; Hoshino, M.; Tanaka, Y.; Akita, M. Near-unity thermally activated delayed fluorescence efficiency in three- and four-coordinate Au(I) complexes with diphosphine ligands. Dalton Trans. 2018, 47, 8229–8239. [Google Scholar] [CrossRef] [PubMed]
- Osawa, M.; Yamayoshi, H.; Hoshino, M.; Tanaka, Y.; Akita, M. Luminescence color alteration induced by trapped solvent molecules in crystals of tetrahedral gold(I) complexes: Near-unity luminescence mixed with thermally activated delayed fluorescence and phosphorescence. Dalton Trans. 2019, 48, 9094–9103. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Wang, H.F.; Ren, Z.G.; Braunstein, P.; Lang, J.P. Fine-Tuning of Luminescence through Changes in Au–S Bond Lengths as a Function of Temperature or Solvent. Inorg. Chem. 2019, 58, 8533–8540. [Google Scholar] [CrossRef] [PubMed]
- Di, D.; Romanov, A.S.; Yang, L.; Richter, J.M.; Rivett, J.P.H.; Jones, S.; Thomas, T.H.; Abdi Jalebi, M.; Friend, R.H.; Linnolahti, M.; et al. High-performance light-emitting diodes based on carbene-metal-amides. Science 2017, 356, 159–163. [Google Scholar] [CrossRef] [Green Version]
- Hamze, R.; Shi, S.; Kapper, S.C.; Muthiah Ravinson, D.S.; Estergreen, L.; Jung, M.C.; Tadle, A.C.; Haiges, R.; Djurovich, P.I.; Peltier, J.L.; et al. “Quick-Silver” from a Systematic Study of Highly Luminescent, Two-Coordinate, d 10 Coinage Metal Complexes. J. Am. Chem. Soc. 2019, 141, 8616–8626. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cestau, J.; Bertrand, B.; Blaya, M.; Jones, G.A.; Penfold, T.J.; Bochmann, M. Synthesis and luminescence modulation of pyrazine-based gold(III) pincer complexes. Chem. Commun. 2015, 51, 16629–16632. [Google Scholar] [CrossRef] [PubMed]
- To, W.P.; Zhou, D.; Tong, G.S.M.; Cheng, G.; Yang, C.; Che, C.M. Highly Luminescent Pincer Gold(III) Aryl Emitters: Thermally Activated Delayed Fluorescence and Solution-Processed OLEDs. Angew. Chem. Int. Ed. 2017, 56, 14036–14041. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, J.C.; Rodríguez, L. Highlights on Gold TADF Complexes. Inorganics 2019, 7, 124. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7100124
Lima JC, Rodríguez L. Highlights on Gold TADF Complexes. Inorganics. 2019; 7(10):124. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7100124
Chicago/Turabian StyleLima, João Carlos, and Laura Rodríguez. 2019. "Highlights on Gold TADF Complexes" Inorganics 7, no. 10: 124. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7100124





