Cytotoxic Gold(I) Complexes with Amidophosphine Ligands Containing Thiophene Moieties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
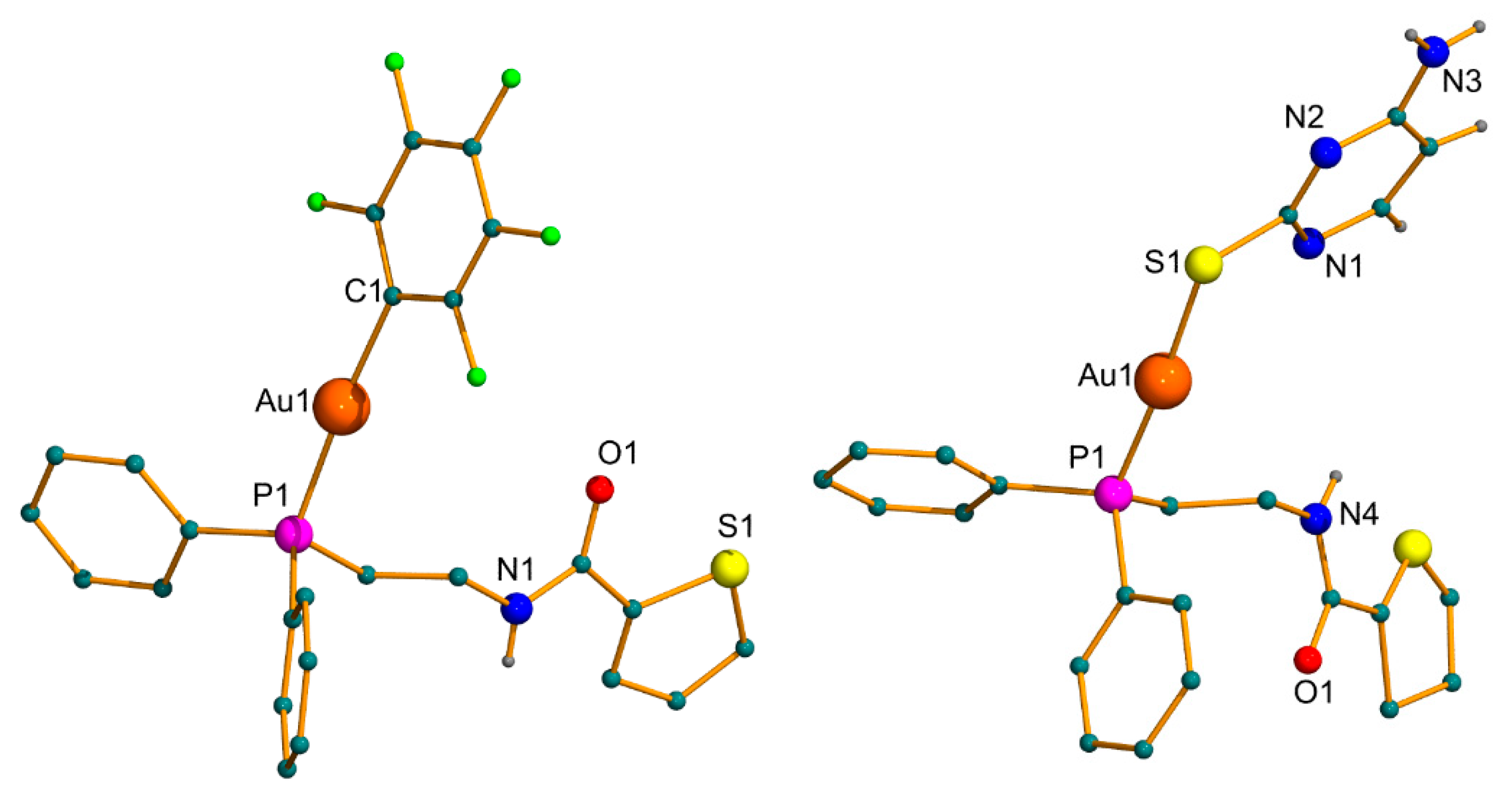
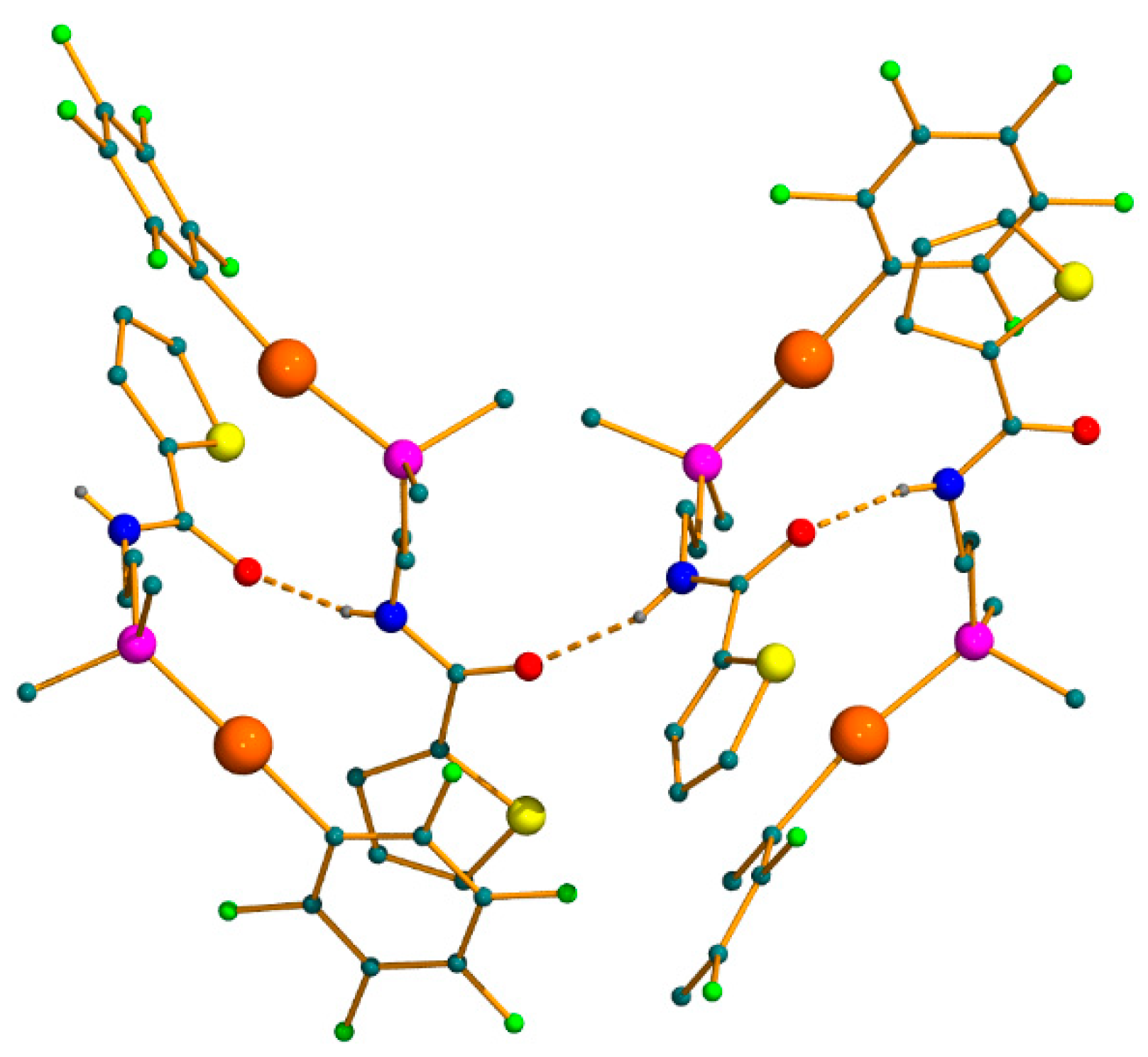
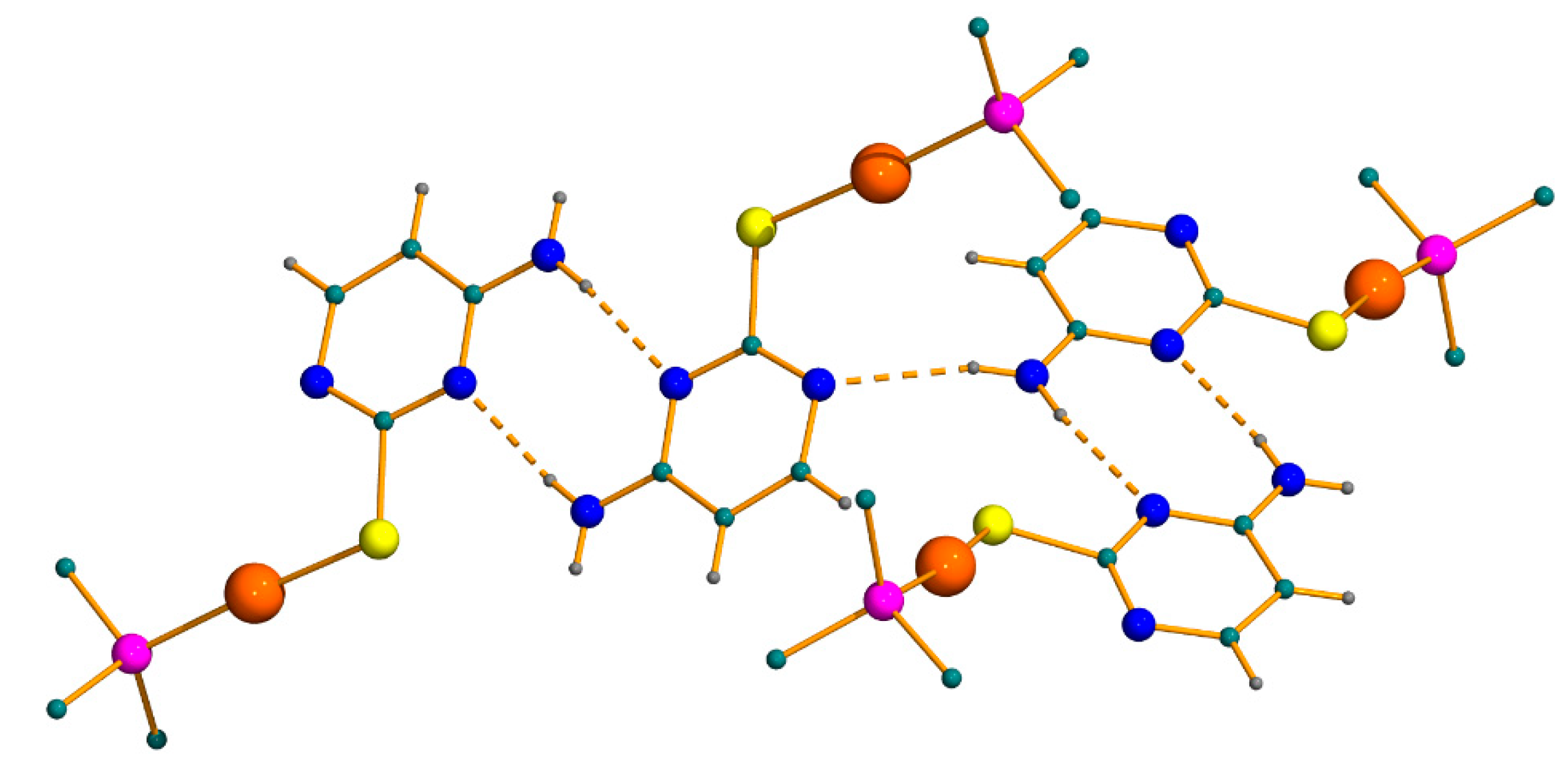
2.2. X-ray Diffraction Studies
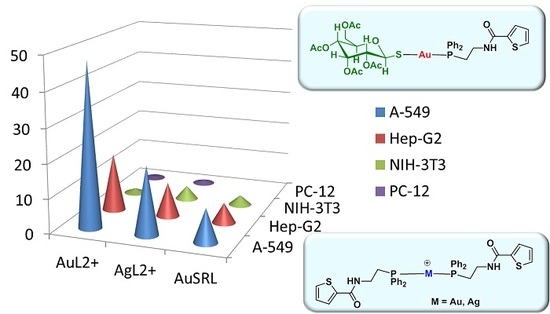
2.3. Biological Studies
3. Experimental Section
3.1. Instrumentation
3.2. Starting Materials
3.3. General Procedure for the Synthesis of Ligand L and Complexes 1–6
3.4. General Procedure for the Synthesis of Phosphinegold(I) Thionucleobase Analogues 7–12
3.5. Cristallography
3.6. Cell Culture
3.7. Cytotoxicity MTT Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosenberg, B.; VanCamp, L.; Trosko, J.E.; Monsour, V.H. Platinum Compounds: A New Class of Potent Antitumour Agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef]
- Lippert, B. Cisplatin: Chemistry and Biochemistry of a Leading Anticancer Drug; Wiley-VCH: Weinheim, Germany, 1999. [Google Scholar]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [Green Version]
- Bergamo, A.; Sava, G. Ruthenium anticancer compounds: Myths and realities of the emerging metal-based drugs. Dalton Trans. 2011, 40, 7817–7823. [Google Scholar] [CrossRef]
- Phase I and II Study of Auranofin in Chronic Lymphocytic Leukemia (CLL). Available online: https://clinicaltrials.gov/ct2/show/NCT01419691 (accessed on 2 September 2018).
- Ott, I. On the medicinal chemistry of gold complexes as anticancer drugs. Coord. Chem. Rev. 2009, 253, 1670–1681. [Google Scholar] [CrossRef]
- Nobili, S.; Mini, E.; Landini, I.; Gabbiani, C.; Casini, A.; Messori, L. Gold compounds as anticancer agents: Chemistry, cellular pharmacology, and preclinical studies. Med. Res. Rev. 2010, 30, 550–580. [Google Scholar] [CrossRef]
- Bertrand, B.B.; Casini, A. A golden future in medicinal inorganic chemistry: The promise of anticancer gold organometallic compounds. Dalton Trans. 2014, 43, 4209–4219. [Google Scholar] [CrossRef]
- Zou, T.; Lum, C.T.; Lok, C.-N.; Zhang, J.-J.; Che, C.M. Chemical biology of anticancer gold(III) and gold(I) complexes. Chem. Soc. Rev. 2015, 44, 8786–8801. [Google Scholar] [CrossRef]
- Barnard, P.T.; Berners-Price, S.J. Targeting the mitochondrial cell death pathway with gold compounds. Coord. Chem. Rev. 2007, 251, 1889–1902. [Google Scholar] [CrossRef]
- Bindoli, A.; Rigobello, M.P.; Scutari, G.; Gabbiani, C.; Casini, A.; Messori, L. Thioredoxin reductase: A target for gold compounds acting as potential anticancer drugs. Coord. Chem. Rev. 2009, 253, 1692–1707. [Google Scholar] [CrossRef]
- Dalla Via, L.; Nardon, C.; Fregona, D. Targeting the ubiquitin-proteasome pathway with inorganic compounds to fight cancer: A challenge for the future. Future Med. Chem. 2012, 4, 525–543. [Google Scholar] [CrossRef]
- Baumann, M.; Baxendale, I.R.; Ley, S.V.; Nikbin, N. An overview of the key routes to the best selling 5-membered ring heterocyclic pharmaceuticals. Beilstein J. Org. Chem. 2011, 7, 442–495. [Google Scholar] [CrossRef] [Green Version]
- Hartman, H.B.; Roehr, J.E.; Rogers, K.L.; Rush, D.K.; Szczepanik, A.M.; Szewczak, M.R.; Wilmot, C.A.; Conway, P.G. 3[4-[1-(6-Fluorobenzo[b]thiophen-3-yl)-4-piperazinyl]butyl]-2,5,5-trimethyl-4-thiazolidinone: A new atypical antipsychotic agent for the treatment of schizophrenia. J. Med. Chem. 1992, 35, 2712–2715. [Google Scholar]
- Bohlman, F.; Zdero, C. Thiophene and Its Derivatives; Gronowitz, S., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 1986; Part 3, pp. 261–323. [Google Scholar]
- Hafez, H.N.; El-Gazzar, A.B.A. Design and synthesis of 3-pyrazolyl-thiophene, thieno[2,3-d]pyrimidines as new bioactive and pharmacological activities. Bioorg. Med. Chem. Lett. 2008, 18, 5222–5227. [Google Scholar] [CrossRef]
- Vogel, V.G.; Costantino, J.P.; Wickerham, D.L.; Cronin, W.M.; Cecchini, R.S.; Atkins, J.N.; Bevers, T.B.; Fehrenbacher, L.; Pajon, E.R., Jr.; Wade, J.L., III; et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. J. Am. Med. Assoc. 2006, 295, 2727–2741. [Google Scholar] [CrossRef]
- Chakrabarti, J.K.; Hotten, T.M.; Tupper, D.E. 2-Methylthienobenzodiazepine. U.S. Patent 5,627,178, 6 May 1997. [Google Scholar]
- Wang, L.; Shen, J.; Tang, Y.; Chen, Y.; Wang, W.; Cai, Z.; Du, Z. Synthetic Improvements in the Preparation of Clopidogrel. Org. Process Res. Dev. 2007, 11, 487–489. [Google Scholar] [CrossRef]
- Gimeno, M.C.; Goitia, H.; Laguna, A.; Luque, M.E.; Villacampa, M.D.; Sepúlveda, C.; Meireles, M. Conjugates of ferrocene with biological compounds. Coordination to gold complexes and antitumoral properties. J. Inorg. Biochem. 2011, 105, 1373–1382. [Google Scholar] [CrossRef] [Green Version]
- Goitia, H.; Nieto, Y.; Villacampa, M.D.; Kasper, C.; Laguna, A.; Gimeno, M.C. Antitumoral Gold and Silver Complexes with Ferrocenyl-Amide Phosphines. Organometallics 2013, 32, 6069–6078. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, A.; Gracia-Fleta, L.; Marzo, I.; Cativiela, C.; Laguna, A.; Gimeno, M.C. Gold(I) thiolates containing amino acid moieties. Cytotoxicity and structure–activity relationship studies. Dalton Trans. 2014, 43, 17054–17066. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Marzo, I.; Cativiela, C.; Laguna, A.; Gimeno, M.C. Highly Cytotoxic Bioconjugated Gold(I) Complexes with Cysteine-Containing Dipeptides. Chem. Eur. J. 2015, 21, 11088–11095. [Google Scholar] [CrossRef] [Green Version]
- Ortego, L.; Meireles, M.; Kasper, C.; Laguna, A.; Villacampa, M.D.; Gimeno, M.C. Group 11 complexes with amino acid derivatives: Synthesis and antitumoral studies. J. Inorg. Biochem. 2016, 156, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Bardají, M.; Jones, P.G.; Laguna, A.; Villacampa, M.D.; Villaverde, N. Synthesis and Structural Characterization of Luminescent Gold(I) Derivatives with an Unsymmetric Diphosphine. Dalton Trans. 2003, 23, 4529–4536. [Google Scholar] [CrossRef]
- Fillat, M.F.; Gimeno, M.C.; Laguna, A.; Latorre, E.; Ortego, L.; Villacampa, M.D. Synthesis, structure and bactericide activity of (aminophosphane)gold(I) thiolate complexes. Eur. J. Inorg. Chem. 2011, 9, 1487–1495. [Google Scholar] [CrossRef]
- Artigas, M.M.; Crespo, O.; Gimeno, M.C.; Jones, P.G.; Laguna, A.; Villacampa, M.D. Synthesis and characterization of [Ag4(µ3-SC2B10H11)2(µ-O3SCF3)2(PPh3)4]: A silver complex with a µ3-thiolate ligand. Inorg. Chem. 1997, 36, 6454–6456. [Google Scholar] [CrossRef]
- Bardají, M.; Crespo, O.; Laguna, A.; Fischer, A.K. Structural characterization of silver(I) complexes [Ag(O3SCF3)(L)] (L = PPh3, PPh2Me, SC4H8) and [AgLn](CF3SO3) (n = 2–4), (L = PPh3, PPh2Me). Inorg. Chim. Acta 2000, 304, 7–16. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, W.Y.; Turner, S.; Ducatman, B.S. Gleevec (STI-571) inhibits lung cancer cell growth (A549) and potentiates the cisplatin effect in vitro. Mol. Cancer 2003, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.; Ma, Z.-Y.; Li, A.; Liu, Y.-H.; Xie, C.-Z.; Qiang, Z.-Y.; Xu, J.-Y. Thiosemicarbazone Cu(II) and Zn(II) complexes as potential anticancer agents: Syntheses, crystal structure, DNA cleavage, cytotoxicity and apoptosis induction activity. J. Inorg. Biochem. 2014, 136, 13–23. [Google Scholar] [CrossRef]
- Suntharalingam, K.; Mendoza, O.; Duarte, A.A.; Mann, D.J.; Vilar, R. A platinum complex that binds non-covalently to DNA and induces cell death via a different mechanism than cisplatin. Metallomics 2013, 5, 514–523. [Google Scholar] [CrossRef]
- Usón, R.; Laguna, A. Polyaryl Derivatives of Gold(I), Silver(I) and Gold(III). In Organometallic Syntheses; King, R.B., Eisch, J.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1986; Volume 3, pp. 322–342. [Google Scholar]
- Usón, R.; Laguna, A.; Navarro, A.; Parish, R.V.; Moore, L.S. Synthesis and reactivity of perchlorate bis(tetrahydrothiophen)gold(I). 197Au Mössbauer spectra of three-coordinate gold(I) complexes. Inorg. Chim. Acta 1986, 112, 205–208. [Google Scholar] [CrossRef]
- Usón, R.; Laguna, A.; Laguna, M.; Jiménez, J.; Gómez, M.P.; Sainz, A.; Jones, P.G. Gold complexes with heterocyclic thiones as ligands. X-Ray structure determination of [Au(C5H5NS)2]ClO4. J. Chem. Soc. Dalton Trans. 1990, 3457–3463. [Google Scholar] [CrossRef]
- CrysAlisPro, Version 1.171.35.11; Agilent Technologies: Yarnton, UK, 2011.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Mosmann, T.J. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]




| IC50 (μM) | ||||
|---|---|---|---|---|
| Complex | A-549 | Hep-G2 | NIH-3T3 | PC-12 |
| L | >100 | >100 | >100 | >100 |
| 1 | 48.9 ± 2.1 | 17.1 ± 1.1 | 1.4 ± 0.09 | 0.5 ± 0.1 |
| 2 | >100 | 16.9 ± 1.2 | 2.8 ± 0.9 | 1.6 ± 0.8 |
| 3 | 10.6 ± 1.4 | 56 ± 3.5 | 39.37 ± 2.2 | 1.6 ± 0.7 |
| 4 | >100 | >100 | >100 | >100 |
| 5 | 20.8 ± 2.1 | 10.0 ± 1.1 | 4.0 ± 0.8 | 1.1 ± 0.6 |
| 6 | 12.5 ± 1.2 | 78.9 ± 2.4 | 20.8 ± 1.8 | 6.9 ± 1.0 |
| 7 | 45.1 ± 2.7 | 5.9 ± 1.7 | 3.0 ± 1.2 | 1.0 ± 0.5 |
| 8 | 24.5 ± 1.8 | 6.7 ± 2.3 | 2.1 ± 0.4 | 0.5 ± 0.2 |
| 9 | >100 | 14.1 ± 2.5 | 6.1 ± 1.9 | 1.3 ± 0.4 |
| 10 | >100 | 15.8 ± 2.4 | 6.3 ± 1.7 | 2.7 ± 1.0 |
| 11 | 9.9 ± 1.4 | 5.9 ± 1.1 | 2.9 ± 0.9 | - |
| 12 | 65.7 ± 3.4 | >100 | 13.7 ± 2.4 | - |
| Cisplatin | 64 | 25 ± 3.1 | 145 ± 13 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goitia, H.; Villacampa, M.D.; Laguna, A.; Gimeno, M.C. Cytotoxic Gold(I) Complexes with Amidophosphine Ligands Containing Thiophene Moieties. Inorganics 2019, 7, 13. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7020013
Goitia H, Villacampa MD, Laguna A, Gimeno MC. Cytotoxic Gold(I) Complexes with Amidophosphine Ligands Containing Thiophene Moieties. Inorganics. 2019; 7(2):13. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7020013
Chicago/Turabian StyleGoitia, Helen, M. Dolores Villacampa, Antonio Laguna, and M. Concepción Gimeno. 2019. "Cytotoxic Gold(I) Complexes with Amidophosphine Ligands Containing Thiophene Moieties" Inorganics 7, no. 2: 13. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7020013