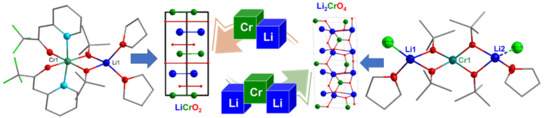
Molecularly Engineered Lithium–Chromium Alkoxide for Selective Synthesis of LiCrO2 and Li2CrO4 Nanomaterials
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of [Li2Cr(OtBu)4Cl(THF)2]n (1)
2.1.1. X-ray Crystallography of Compound 1
2.1.2. Electron Paramagnetic Resonance Spectroscopy (EPR) of Compound 1
2.2. Synthesis of [LiCr(OtBu)2(PyCH=COCF3)2(THF)2] (2)
2.2.1. X-ray Crystallography of Compound 2
2.2.2. Thermogravimetric Analysis (TGA) of Compounds 1
2.3. Material Synthesis
3. Materials and Methods
3.1. Precursor Synthesis
3.2. Synthesis of LiCrO2 Nanoparticles
3.3. Synthesis of Li2CrO4 Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Hu, P.; Long, Y.; Liu, Z.; He, X. Recent advances in ternary two-dimensional materials: Synthesis, properties and applications. J. Mater. Chem. A. 2017, 5, 22855–22876. [Google Scholar] [CrossRef]
- Li, G.; Höpfner, P.; Schäfer, J.; Blumenstein, C.; Meyer, S.; Bostwick, A.; Rotenberg, E.; Claessen, R.; Hanke, W. Magnetic order in a frustrated two-dimensional atom lattice at a semiconductor surface. Nat. Commun. 2013, 4, 1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazin, I.I. Electronic structure and magnetism in the frustrated antiferromagnet LiCrO2: First-principles calculations. Phys. Rev. B 2007, 75, 094407. [Google Scholar] [CrossRef]
- Alexander, L.K.; Büttgen, N.; Nath, R.; Mahajan, A.V.; Loidl, A. 7Li NMR studies on the triangular lattice system LiCrO2. Phys. Rev. B 2007, 76, 064429. [Google Scholar] [CrossRef]
- Moessner, R.; Ramirez, A.P. Geometrical frustration. Phys. Today 2006, 59, 24. [Google Scholar] [CrossRef]
- Cao, M.; Wang, T.; Shadike, Z.; Nam, K.; Zhou, Y.; Fu, Z. Reversible Multi-Electron Transfer of Cr2.8+/Cr4.4+ in O3-Type Layered Na0.66Fe1/3Cr1/3Ti1/3O2 for Sodium-Ion Batteries. J. Electrochem. Soc. 2018, 165, A565–A574. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Song, D.; Shi, X.; Tang, X.; Zhang, H.; Zhang, L. Unravelling the Structure and Electrochemical Performance of Li–Cr–Mn–O Cathodes: From Spinel to Layered. ACS Appl. Mater. Interfaces 2018, 10, 8827–8835. [Google Scholar] [CrossRef]
- Xu, M.; Fei, L.; Zhang, W.; Li, T.; Lu, W.; Zhang, N.; Lai, Y.; Zhang, Z.; Fang, J.; Zhang, K.; et al. Tailoring anisotropic Li-Ion transport tunnels on orthogonally arranged Li-rich layered oxide nanoplates toward high-performance Li-ion batteries. Nano Lett. 2017, 17, 1670–1677. [Google Scholar] [CrossRef]
- Benedek, R.; Iddir, H. Simulation of First-Charge Oxygen-Dimerization and Mn-Migration in Li-Rich Layered Oxides xLi2MnO3·(1−x) LiMO2 and Implications for Voltage Fade. J. Phys. Chem. C 2017, 121, 6492–6499. [Google Scholar] [CrossRef]
- Chisholm, M.H.; Eilerts, N.W.; Huffman, J.C.; Iyer, S.S.; Pacold, M.; Phomphrai, K. Molecular design of single-site metal alkoxide catalyst precursors for ring-opening polymerization reactions leading to polyoxygenates. 1. Polylactide formation by achiral and chiral magnesium and zinc alkoxides, (η3-L) MOR, where L = trispyrazolyl-and trisindazolylborate ligands. J. Am. Chem. Soc. 2000, 122, 11845–11854. [Google Scholar]
- Ovitt, T.M.; Coates, G.W. Stereoselective ring-opening polymerization of rac-lactide with a single-site, racemic aluminum alkoxide catalyst: Synthesis of stereoblock poly(lactic acid). J. Polym. Sci. A Poly. Chem. 2000, 38, 4686–4692. [Google Scholar] [CrossRef]
- Mishra, S.; Agarwal, R.; Singh, A. Four novel classes of heterobimetallic isopropoxides of chromium(III). Transit. Met. Chem. 2002, 27, 712–715. [Google Scholar] [CrossRef]
- Agarwal, S.K.; Mehrotra, R.C. Synthesis and physico-chemical studies of bimetallic alkoxy derivatives of chromium(III) with niobium(V) and tantalum(V). Inorganica Chim. Acta 1986, 112, 177–181. [Google Scholar] [CrossRef]
- Mahendra, K.N.; Mehrotra, R.C. Preparation and Reactions of Chromium(III) alkoxides. Synth. React. Inorg. Met.-Org. Chem. 1990, 20, 963–973. [Google Scholar] [CrossRef]
- Brown, D.A.; Cunningham, D.; Glass, W.K. Studies of chromium(III) alkoxides. J. Chem. Soc. A 1968, 0, 1563–1568. [Google Scholar] [CrossRef]
- Edema, J.J.; Gambarotta, S.; Smeets, W.J.; Spek, A.L. Polymetallic chromium alkoxides: Synthesis and crystal structures of (iso-PrO)8Cr2Na4(THF)4 and (. mu. 3-OPh)10Cr4 (. mu. 3-O)3Na4 (TMEDA)4. Inorg. Chem. 1991, 30, 1380–1384. [Google Scholar] [CrossRef]
- Tóth, S.; Wehinger, B.; Rolfs, K.; Birol, T.; Stuhr, U.; Takatsu, H.; Kimura, K.; Kimura, T.; Rønnow, H.M.; Rüegg, C. Electromagnon dispersion probed by inelastic X-ray scattering in LiCrO2. Nat. Commun. 2016, 7, 13547. [Google Scholar] [CrossRef]
- Garg, A.; Errandonea, D.; Pellicer-Porres, J.; Martinez-Garcia, D.; Kesari, S.; Rao, R.; Bettinelli, M. LiCrO2 Under Pressure: In-Situ Structural and Vibrational Studies. Crystals 2019, 9, 2. [Google Scholar] [CrossRef]
- Feng, G.X.; Li, L.F.; Liu, J.Y.; Liu, N.; Li, H.; Yang, X.Q.; Huang, X.J.; Chen, L.Q.; Nam, K.W.; Yoon, W.S. Enhanced electrochemical lithium storage activity of LiCrO2 by size effect. J. Mater. Chem. 2009, 19, 2993–2998. [Google Scholar] [CrossRef]
- Zopes, D.; Hegemann, C.; Schläfer, J.; Tyrra, W.; Mathur, S. Single-Source Precursors for Alloyed Gold–Silver Nanocrystals-A Molecular Metallurgy Approach. Inorg. Chem. 2015, 54, 3781–3787. [Google Scholar] [CrossRef]
- Garg, R.; Hegemann, C.; Mathur, S. Heterobimetallic Alkoxides [CdIIMV (OiPr)7]2 (M = Nb, Ta) as Potential Precursors to Pyrochlore Cd2M2O7. Eur. J. Inorg. Chem. 2017, 2017, 3383–3389. [Google Scholar] [CrossRef]
- Jamil, A.; Schläfer, J.; Gönüllü, Y.; Lepcha, A.; Mathur, S. Precursor-derived rare earth metal pyrochlores: Nd2Sn2O7 nanofibers and thin films as efficient photoabsorbers. Cryst. Growth Des. 2016, 16, 5260–5267. [Google Scholar] [CrossRef]
- Barley, H.R.L.; Kennedy, A.R.; Mulvey, R.E. A mixed iron(III)/lithium alkoxide. Acta Crystallogr. C 2005, 61, m346–m347. [Google Scholar] [CrossRef] [PubMed]
- Mäntymäki, M.; Ritala, M.; Leskelä, M. Double metal alkoxides of lithium: Synthesis, structure and applications in materials chemistry. Coord. Chem. Rev. 2012, 256, 854–877. [Google Scholar] [CrossRef]
- Bonomo, R.P.; Di Bilio, A.J.; Riggi, F. EPR investigation of chromium(III) complexes: Analysis of their frozen solution and magnetically dilute powder spectra. Chem. Phys. 1991, 151, 323–333. [Google Scholar] [CrossRef]
- Bradley, D.C. Metal alkoxides as precursors for electronic and ceramic materials. Chem. Rev. 1989, 89, 1317–1322. [Google Scholar] [CrossRef]
- Ojelere, O.; Graf, D.; Ludwig, T.; Vogt, N.; Klein, A.; Mathur, S. Reductive transformation of V(III) precursors into vanadium(II) oxide nanowires. Dalton Trans. 2018, 47, 6842–6849. [Google Scholar] [CrossRef]
- McIlvried, K.E.; McCarthy, G.J. ICDD: 24-0600; Penn State University, University Park: State College, PA, USA, 1972. [Google Scholar]
- Herres, N.; Klapper, H. ICDD: 31-0715; Institut for Kristallographie, Technische Hochschule: Aachen, Germany, 1979. [Google Scholar]
- Tanaka, M.; Kageyama, T.; Sone, H.; Yoshida, S.; Okamoto, D.; Watanabe, T. Synthesis of Lithium Metal oxide nanoparticles by induction thermal plasmas. Nanomaterials 2016, 6, 60. [Google Scholar] [CrossRef]
- X-AREA; Stoe & Cie GmbH: Darmstadt, Germany, 2005.
- X-RED32, 1.31; Stoe & Cie GmbH: Darmstadt, Germany, 2005.
- X-SHAPE, 1.06; Stoe & Cie GmbH: Darmstadt, Germany, 1999.
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M.C.; Polidori, G.; Camalli, M. SIRPOW. 92—A program for automatic solution of crystal structures by direct methods optimized for powder data. J. Appl. Cryst. 1994, 27, 435. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojelere, O.; Graf, D.; Mathur, S. Molecularly Engineered Lithium–Chromium Alkoxide for Selective Synthesis of LiCrO2 and Li2CrO4 Nanomaterials. Inorganics 2019, 7, 22. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7020022
Ojelere O, Graf D, Mathur S. Molecularly Engineered Lithium–Chromium Alkoxide for Selective Synthesis of LiCrO2 and Li2CrO4 Nanomaterials. Inorganics. 2019; 7(2):22. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7020022
Chicago/Turabian StyleOjelere, Olusola, David Graf, and Sanjay Mathur. 2019. "Molecularly Engineered Lithium–Chromium Alkoxide for Selective Synthesis of LiCrO2 and Li2CrO4 Nanomaterials" Inorganics 7, no. 2: 22. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7020022