1H NMR Relaxometric Analysis of Paramagnetic Gd2O3:Yb Nanoparticles Functionalized with Citrate Groups
Abstract
:1. Introduction
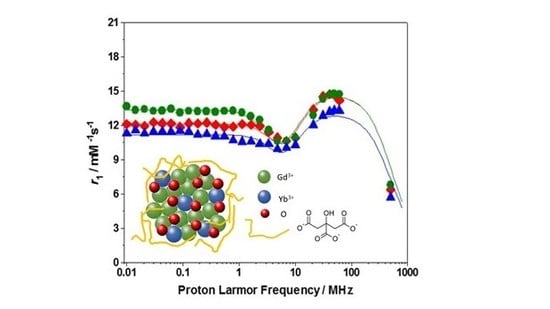
2. Results and Discussion
3. Materials and Methods
Characterisation Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Doan, B.-T.; Meme, S.; Beloeil, J.-C. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging; Merbach, A., Helm, L., Toth, E., Eds.; John Wiley & Sons: Chichester, UK, 2013; pp. 1–23. [Google Scholar]
- Wahsner, J.; Gale, E.M.; Rodriguez-Rodriguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, S.; Blackburn, O.A. The Chemistry of Molecular Imaging; Long, N., Wong, W.-T., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 179–197. [Google Scholar]
- Hanaoka, K.; Lubag, A.J.; Castillo-Muzquiz, A.; Kodadek, T.; Sherry, A.D. The detection limit of a Gd3+-based T1 agent is substantially reduced when targeted to a protein microdomain. Magn. Reson. Imaging 2008, 26, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Carniato, F.; Tei, T.; Dastrú, W.; Marchese, L.; Botta, M. Relaxivity modulation in Gd-functionalised mesoporous silicas. Chem. Commun. 2009, 1246–1248. [Google Scholar] [CrossRef] [PubMed]
- Carniato, F.; Tei, L.; Cossi, M.; Marchese, L.; Botta, M. A Chemical Strategy for the Relaxivity Enhancement of GdIII Chelates Anchored on Mesoporous Silica Nanoparticles. Chem. Eur. J. 2010, 16, 10727–10734. [Google Scholar] [CrossRef] [PubMed]
- Carniato, F.; Tei, L.; Arrais, A.; Marchese, L.; Botta, M. Selective Anchoring of GdIII Chelates on the External Surface of Organo-Modified Mesoporous Silica Nanoparticles: A New Chemical Strategy to Enhance Relaxivity. Chem. Eur. J. 2013, 19, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Carniato, F.; Tei, L.; Botta, M. Gd-Based Mesoporous Silica Nanoparticles as MRI Probes. Eur. J. Inorg. Chem. 2018, 4936–4954. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Davies, G.-L.; Davis, J.J. High signal contrast gating with biomodified Gd doped mesoporous nanoparticles. Chem. Commun. 2013, 49, 60–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchoucha, M.; C.-Gaudreault, R.; Fortin, M.-A.; Kleitz, F. Mesoporous Silica Nanoparticles: Selective Surface Functionalization for Optimal Relaxometric and Drug Loading Performances. Adv. Funct. Mater. 2014, 24, 5911–5923. [Google Scholar] [CrossRef]
- Dong, H.; Du, S.-R.; Zheng, X.-Y.; Lyu, G.-M.; Sun, L.-D.; Li, L.-D.; Zhang, P.-Z.; Zhang, C.; Yan, C.-H. Lanthanide Nanoparticles: From Design toward Bioimaging and Therapy. Chem. Rev. 2015, 115, 10725–10815. [Google Scholar] [CrossRef] [PubMed]
- Guillet-Nicolas, R.; Jean, N.; Bridot, L.; Seo, Y.; Fortin, M.-A.; Kleitz, F. Enhanced Relaxometric Properties of MRI “Positive” Contrast Agents Confined in Three-Dimensional Cubic Mesoporous Silica Nanoparticles. Adv. Funct. Mater. 2011, 21, 4653–4662. [Google Scholar] [CrossRef]
- Johnson, N.J.J.; Oakden, W.; Stanisz, G.J.; Prosser, R.S.; van Veggel, F.C.J.M. Size-Tunable, Ultrasmall NaGdF4 Nanoparticles: Insights into Their T1 MRI Contrast Enhancement. Chem. Mater. 2011, 23, 3714–3722. [Google Scholar] [CrossRef]
- Ahmad, M.W.; Kim, C.R.; Baeck, J.S.; Chang, Y.; Kim, T.J.; Bae, J.E.; Chaed, K.S.; Lee, G.H. Bovine Serum Albumin (BSA) and Cleaved-BSA Conjugated Ultrasmall Gd2O3 Nanoparticles: Synthesis, Characterization, and Application to MRI Contrast Agents. Colloids Surf. A 2014, 450, 67–75. [Google Scholar] [CrossRef]
- Ahren, M.; Selegaard, L.; Soederlind, F.; Linares, M.; Kauczor, J.; Norman, P.; Kaell, P.-O.; Uvdal, K. A Simple Polyol-free Synthesis Route to Gd2O3 Nanoparticles for MRI Applications: An Experimental and Theoretical Study. J. Nanopart. Res. 2012, 14, 1006–1022. [Google Scholar] [CrossRef]
- Cho, M.; Sethi, R.; Ananta narayanan, J.S.; Lee, S.S.; Benoit, D.N.; Taheri, N.; Decuzzi, P.; Colvin, V.L. Gadolinium Oxide Nanoplates with High Longitudinal Relaxivity for Magnetic Resonance Imaging. Nanoscale 2014, 6, 13637–13645. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, M.; Miao, F.; Ma, J.; Shen, H.; Jia, N. Regulation of Multifunctional Mesoporous Core–shell Nanoparticles with Luminescence and Magnetic Properties for Biomedical Applications. J. Mater. Chem. B 2014, 2, 2265–2275. [Google Scholar] [CrossRef]
- Liu, J.; Tian, X.; Luo, N.; Yang, C.; Xiao, J.; Shao, Y.; Chen, X.; Yang, G.; Chen, D.; Li, L. Sub-10 nm Monoclinic Gd2O3:Eu3+ Nanoparticles as Dual-Modal Nanoprobes for Magnetic Resonance and Fluorescence Imaging. Langmuir 2014, 30, 13005–13013. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, M.; Yang, C.; Liu, J.; Luo, N.Q.; Yang, G.W.; Chen, D.H.; Li, L. Terbium-doped Gadolinium Oxide Nanoparticles Prepared by Laser Ablation in Liquid for Use as a Fluorescence and Magnetic Resonance Imaging Dual-modal Contrast Agent. Phys. Chem. Chem. Phys. 2015, 17, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Petoral, R.M.; Soederlind, F.; Klasson, A.; Suska, A.; Fortin, M.A.; Abrikossova, N.; Selegaard, L.; Kaell, P.-O.; Engstroem, M.; Uvdal, K. Synthesis and Characterization of Tb3+-Doped Gd2O3 Nanocrystals: A Bifunctional Material with Combined Fluorescent Labeling and MRI Contrast Agent Properties. J. Phys. Chem. C 2009, 113, 6913–6920. [Google Scholar] [CrossRef]
- Luo, N.; Yang, C.; Tian, X.; Xiao, J.; Liu, J.; Chen, F.; Zhang, D.; Xu, D.; Zhang, Y.; Yang, G.; et al. A General top-down Approach to Synthesize Rare Earth Doped-Gd2O3 Nanocrystals as Dualmodal Contrast Agents. J. Mater. Chem. B 2014, 2, 5891–5897. [Google Scholar] [CrossRef]
- Liu, Z.; Pu, F.; Liu, J.; Jiang, L.; Yuan, Q.; Li, Z.; Ren, J.; Qu, X. PEGylated Hybrid Ytterbia Nanoparticles as High-Performance Diagnostic Probes for in Vivo Magnetic resonance and X-Ray Computed Tomography Imaging with Low Systemic Toxicity. Nanoscale 2013, 5, 4252–4261. [Google Scholar] [CrossRef] [PubMed]
- Carniato, F.; Thangavel, K.; Tei, L.; Botta, M. Structure and dynamics of the hydration shells of citrate-coated GdF3 nanoparticles. J. Mater. Chem. B 2013, 1, 2442–2446. [Google Scholar] [CrossRef]
- Söderlind, F.; Pedersen, H.; Petoral, R.M., Jr.; Käll, P.-O.; Uvdal, K. Synthesis and characterisation of Gd2O3 nanocrystals functionalised by organic acids. J. Colloid Interface Sci. 2005, 288, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Caruntu, D.; Remond, Y.; Chou, N.H.; Jun, M.-J.; Caruntu, G.; He, J.; Goloverda, G.; O’Connor, C.; Kolesnichenko, V. Reactivity of 3d Transition Metal Cations in Diethylene Glycol Solutions. Synthesis of Transition Metal Ferrites with the Structure of Discrete Nanoparticles Complexed with Long-Chain Carboxylate Anions. Inorg. Chem. 2002, 41, 6137–6146. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Peng, E.; Zheng, B.; Li, S.F.Y.; Xue, J.M. Synthesis of Water-Dispersible Gd2O3/GO Nanocomposites with Enhanced MRI T1 Relaxivity. J. Phys. Chem. C 2015, 119, 23735–23742. [Google Scholar] [CrossRef]
- Faucher, L.; Guay-Bégin, A.A.; Lagueux, J.; Côté, M.-F.; Petitclerc, E.; Fortin, M.-A. Ultra-small gadolinium oxide nanoparticles to image brain cancer cells in vivo with MRI. Contrast Media Mol. Imaging 2011, 6, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef] [PubMed]
- Aime, S.; Geninatti Crich, S.; Gianolio, E.; Giovenzana, G.B.; Tei, L.; Terreno, E. High sensitivity lanthanide(III) based probes for MR-medical imaging. Coord. Chem. Rev. 2006, 250, 1562–1579. [Google Scholar] [CrossRef]
- Bridot, J.-L.; Faure, A.-C.; Laurent, S.; Rivière, C.; Billotey, C.; Hiba, B.; Janier, M.; Josserand, V.; Coll, J.-L.; Vander Elst, L.; et al. Hybrid Gadolinium Oxide Nanoparticles: Multimodal Contrast Agents for in Vivo Imaging. J. Am. Chem. Soc. 2007, 129, 5076–5084. [Google Scholar] [CrossRef] [PubMed]
- Botta, M. Second Coordination Sphere Water Molecules and Relaxivity of Gadolinium(III) Complexes: Implications for MRI Contrast Agents. Eur. J. Inorg. Chem. 2000, 399–407. [Google Scholar] [CrossRef]
- Carniato, F.; Tei, L.; Phadngam, S.; Isidoro, C.; Botta, M. NaGdF4 Nanoparticles Coated with Functionalised Ethylenediaminetetraacetic Acid as Versatile Probes for Dual Optical and Magnetic Resonance Imaging. ChemPlusChem 2015, 80, 503–510. [Google Scholar] [CrossRef]
- Helm, L.; Morrow, J.R.; Bond, C.J.; Carniato, F.; Botta, M.; Braun, M.; Baranyai, Z.; Pujales-Paradela, R.; Regueiro-Figueroa, M.; Esteban-Gómez, D.; et al. Contrast Agents for MRI: Experimental Methods; Pierre, V.C., Allen, M.J., Eds.; RSC: London, UK, 2018; pp. 11–242. [Google Scholar]
- Solomon, I.; Bloembergen, N. Nuclear Magnetic Interactions in the HF Molecule. J. Chem. Phys. 1956, 25, 261. [Google Scholar] [CrossRef]
- Freed, J.H. Dynamic effects of pair correlation functions on spin relaxation by translational diffusion in liquids. II. Finite jumps and independent T1 processes. J. Chem. Phys. 1978, 68, 4034. [Google Scholar] [CrossRef]





| Samples | r140 (mM−1 s−1) | r160 (mM−1 s−1) | r2/r1 (40 MHz) | r2/r1 (60 MHz) |
|---|---|---|---|---|
| Gd2O3 | 14.7 | 14.2 | 1.35 | 1.60 |
| Gd2O3:Yb (LL) | 14.7 | 14.7 | 1.39 | 1.92 |
| Gd2O3:Yb (HL) | 13.1 | 13.2 | 1.43 | 1.64 |
| Samples | Δ2 (1019 s−2) | τV (ps) | q | τR (ns) | q′ | τR′ (ns) |
|---|---|---|---|---|---|---|
| Gd2O3 | 2.83 ± 0.17 | 37.0 ± 2.3 | 1 | 0.35 + 0.01 | 1 | 0.20 + 0.01 |
| Gd2O3:Yb (LL) | 2.77 ± 0.13 | 32.6 ± 1.6 | 1 | 0.35 + 0.01 | 1 | 0.20 + 0.02 |
| Gd2O3:Yb (HL) | 2.78 ± 0.21 | 32.8 ± 2.5 | 1 | 0.35 + 0.01 | 0.75 | 0.17 + 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carniato, F.; Gatti, G. 1H NMR Relaxometric Analysis of Paramagnetic Gd2O3:Yb Nanoparticles Functionalized with Citrate Groups. Inorganics 2019, 7, 34. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7030034
Carniato F, Gatti G. 1H NMR Relaxometric Analysis of Paramagnetic Gd2O3:Yb Nanoparticles Functionalized with Citrate Groups. Inorganics. 2019; 7(3):34. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7030034
Chicago/Turabian StyleCarniato, Fabio, and Giorgio Gatti. 2019. "1H NMR Relaxometric Analysis of Paramagnetic Gd2O3:Yb Nanoparticles Functionalized with Citrate Groups" Inorganics 7, no. 3: 34. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7030034