Laser Ablation Inductively Coupled Plasma Spectrometry: Metal Imaging in Experimental and Clinical Wilson Disease
Abstract
:1. Introduction
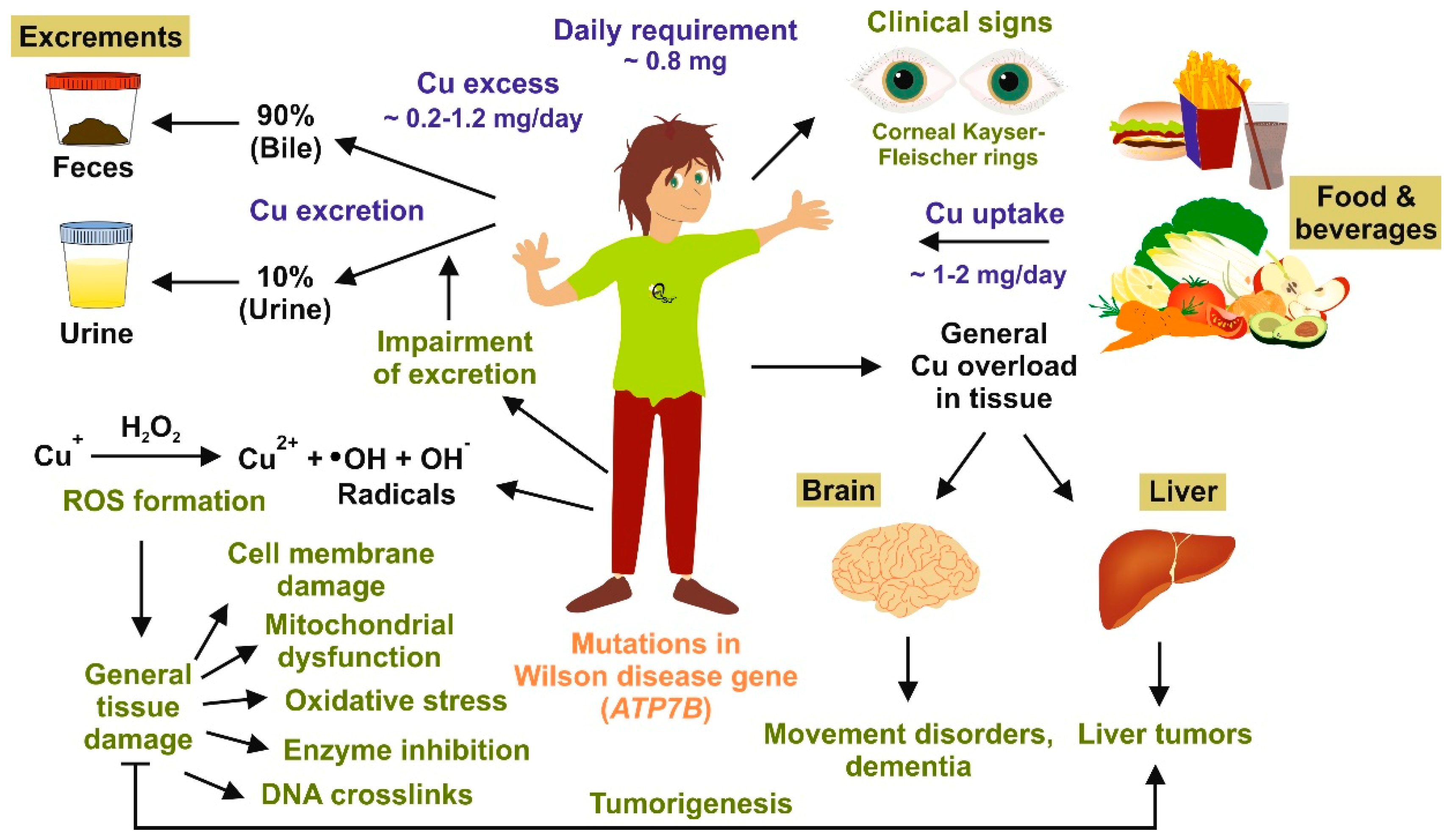
2. Copper Homeostasis and Overload
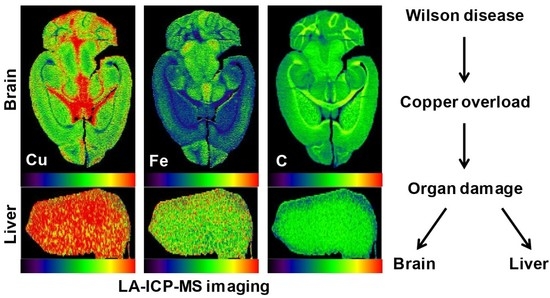
3. Copper Distribution in Wilson Disease Tissue
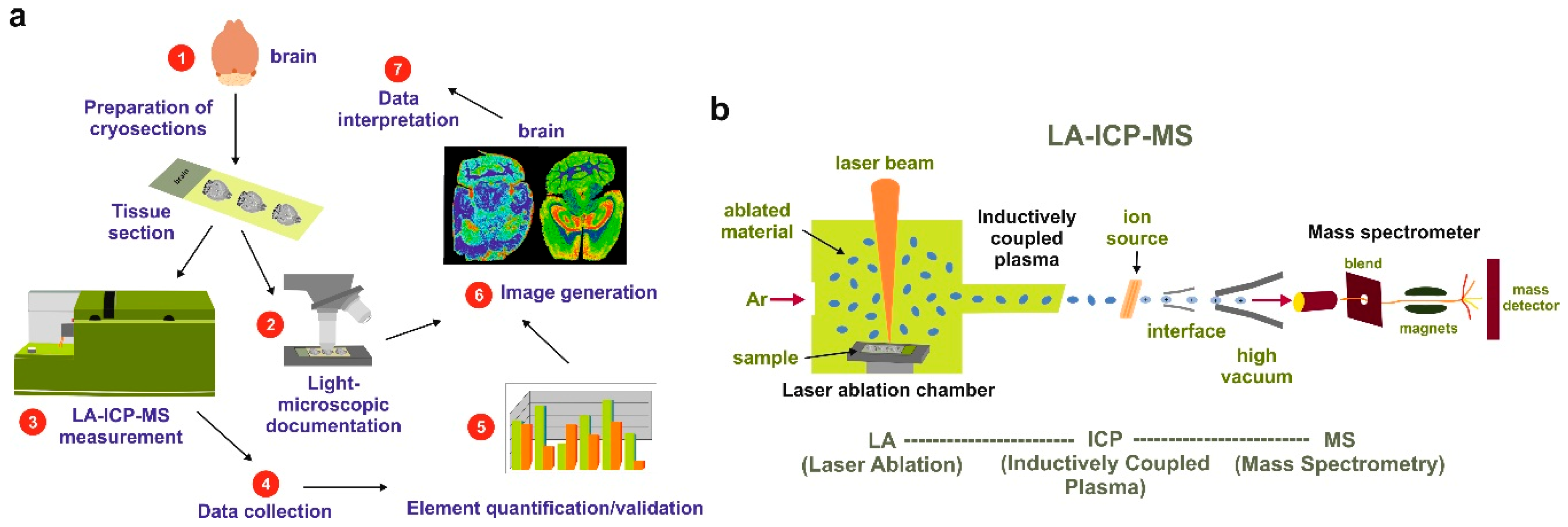
4. Laser Ablation Inductively Coupled Plasma Spectrometry Imaging
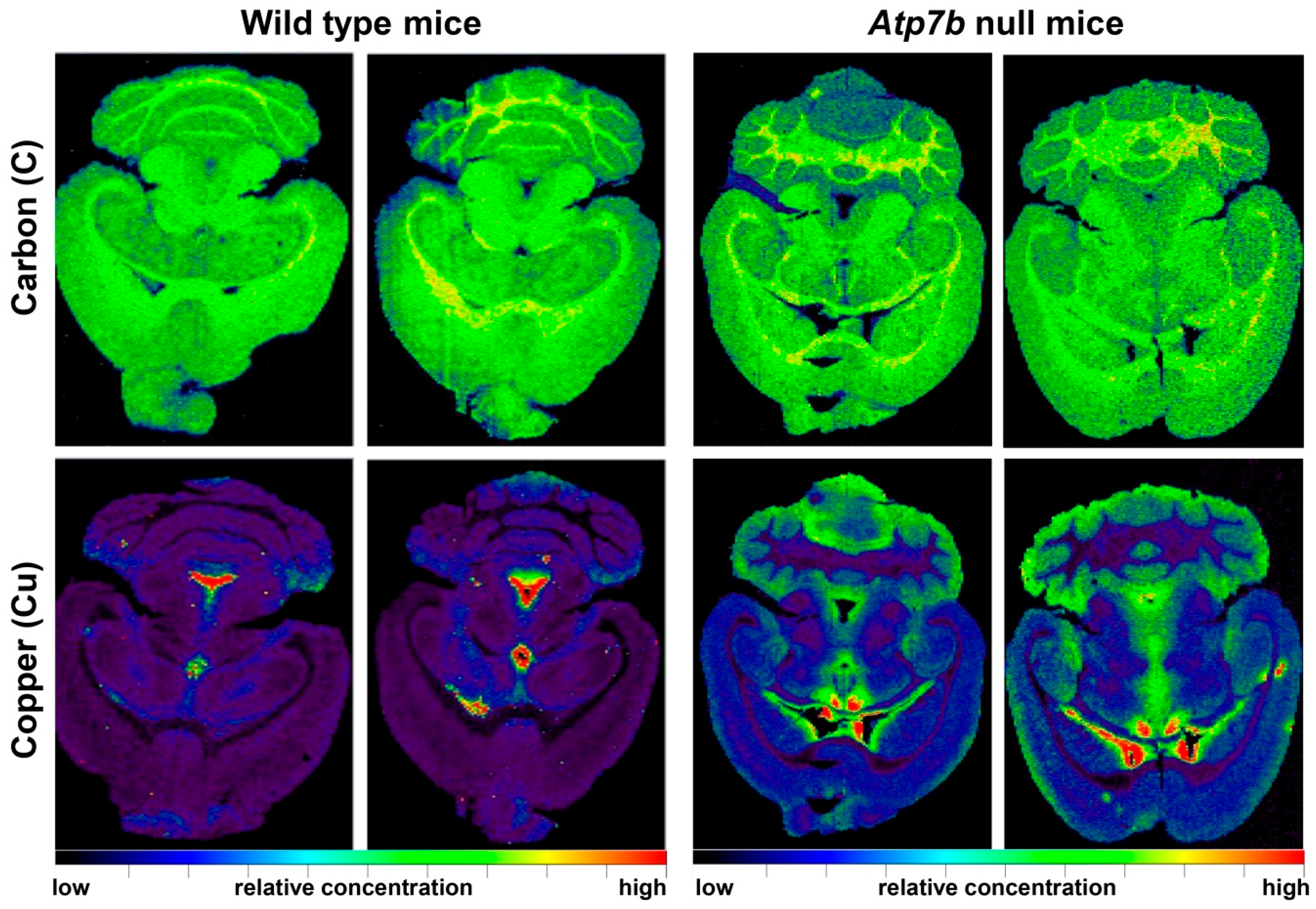
5. Wilson Disease Studies Using LA-ICP-MSI
6. Translational Aspects of LA-ICP-MSI
7. Materials and Methods
7.1. Images and Pictures
7.2. Animals
7.3. Human Samples
7.4. LA-ICP-MSI Measurements
7.5. Visualization of Data
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Merle, U.; Weiskirchen, R. Wilson’s disease: An inherited, silent, copper intoxication disease. Emj Neurol. 2016, 4, 74–83. [Google Scholar]
- Reilly, M.; Daly, L.; Hutchinson, M. An epidemiological study of Wilson’s disease in the Republic of Ireland. J. Neurol. Neurosurg. Psychiatry 1993, 56, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Cocoş, R.; Şendroiu, A.; Schipor, S.; Bohîlţea, L.C.; Şendroiu, I.; Raicu, F. Genotype-phenotype correlations in a mountain population community with high prevalence of Wilson’s disease: Genetic and clinical homogeneity. PLoS ONE 2014, 9, e98520. [Google Scholar] [CrossRef]
- European Association for Study of Liver. EASL Clinical Practice Guidelines: Wilson’s disease. J. Hepatol. 2012, 56, 671–685. [Google Scholar] [CrossRef] [Green Version]
- Stremmel, W.; Merle, U.; Weiskirchen, R. Clinical features of Wilson disease. Ann. Transl. Med. 2019. [Google Scholar] [CrossRef]
- Medici, V.; LaSalle, J.M. Genetics and epigenetic factors of Wilson disease. Ann. Transl. Med. 2019. [Google Scholar] [CrossRef]
- Roberts, E.A.; Schilsky, M.L.; American Association for Study of Liver Diseases (AASLD). Diagnosis and treatment of Wilson disease: An update. Hepatology 2008, 47, 2089–2111. [Google Scholar] [CrossRef] [Green Version]
- Palumbo, C.S.; Schilsky, M.L. Clinical practice guidelines in Wilson disease. Ann. Transl. Med. 2019. [Google Scholar] [CrossRef]
- Litwin, T.; Dzieżyc, K.; Członkowska, A. Wilson disease—Treatment perspectives. Ann. Transl. Med. 2019. [Google Scholar] [CrossRef]
- Medici, V.; Huster, D. Animal models of Wilson disease. Handb. Clin. Neurol. 2017, 142, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Reed, E.; Lutsenko, S.; Bandmann, O. Animal models of Wilson disease. J. Neurochem. 2018, 146, 356–373. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liu, L.; Chang, Q.; Xing, F.; Ma, Z.; Fang, Z.; Zhou, J.; Fu, L.; Wang, H.; Huang, X.; et al. Production of Wilson disease model rabbits with homology-directed precision point mutations in the ATP7B gene using the CRISPR/Cas9 system. Sci. Rep. 2018, 8, 1332. [Google Scholar] [CrossRef]
- Bourassa, M.W.; Miller, L.M. Metal imaging in neurodegenerative diseases. Metallomics 2012, 4, 721–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Susnea, I.; Weiskirchen, R. Trace metal imaging in diagnostic of hepatic metal disease. Mass Spectrom. Rev. 2016, 35, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Berghe, P.V.; Klomp, L.W. New developments in the regulation of intestinal copper absorption. Nutr. Rev. 2009, 67, 658–672. [Google Scholar] [CrossRef]
- Pierson, H.; Muchenditsi, A.; Kim, B.E.; Ralle, M.; Zachos, N.; Huster, D.; Lutsenko, S. The function of ATPase copper transporter ATP7B in intestine. Gastroenterology 2018, 154, 168–180. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Popiołek, Ł.; Kocot, J. The many “faces” of copper in medicine and treatment. Biometals 2014, 27, 611–621. [Google Scholar] [CrossRef] [Green Version]
- Ogra, Y.; Aoyama, M.; Suzuki, K.T. Protective role of metallothionein against copper depletion. Arch. Biochem. Biophys. 2006, 451, 112–118. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Y.; Lin, Z.; Han, M.; Cheng, H. Altered serum copper homeostasis suggests higher oxidative stress and lower antioxidant capability in patients with chronic hepatitis B. Medicine 2018, 97, e11137. [Google Scholar] [CrossRef]
- Kathawala, M.; Hirschfield, G.M. Insights into the management of Wilson’s disease. Ther. Adv. Gastroenterol. 2017, 10, 889–905. [Google Scholar] [CrossRef]
- Ferenci, P. Wilson’s Disease. Clin. Gastroenterol. Hepatol. 2005, 3, 726–733. [Google Scholar] [CrossRef]
- Hermann, W. Classification and differential diagnosis of Wilson’s disease. Ann. Transl. Med. 2019. [Google Scholar] [CrossRef]
- Faa, G.; Nurchi, V.; Demelia, L.; Ambu, R.; Parodo, G.; Congiu, T.; Sciot, R.; Van Eyken, P.; Silvagni, R.; Crisponi, G. Uneven hepatic copper distribution in Wilson’s disease. J. Hepatol. 1995, 22, 303–308. [Google Scholar] [CrossRef]
- Porter, H. Tissue copper proteins in Wilson’s disease. Intracellular distribution and chormatogrphic fractionation. Arch. Neurol. 1964, 11, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Goldfischer, S.; Popper, H.; Sternlieb, I. The significance of variations in the distribution of copper in liver disease. Am. J. Pathol. 1980, 99, 715–730. [Google Scholar] [PubMed]
- Pilloni, L.; Lecca, S.; Van Eyken, P.; Flore, C.; Demelia, L.; Pilleri, G.; Nurchi, A.M.; Farci, A.M.; Ambu, R.; Callea, F.; et al. Value of histochemical stains for copper in the diagnosis of Wilson’s disease. Histopathology 1998, 33, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Uerlings, R.; Weiskirchen, R. An at-a-glance visualization of regional metal accretion in tissue samples by image overlay in laser ablation inductively coupled plasma mass spectrometry. In Laser Ablation: Advances in Research and Applications Is Approaching; Bellucci, C., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2017; pp. 87–113. [Google Scholar]
- Bandura, D.R.; Baranov, V.I.; Tanner, S.D. Reaction chemistry and collisional processes in multiple devices for resolving isobaric interferences in ICP-MS. Freseniusj. Anal. Chem. 2001, 370, 454–470. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Weiskirchen, S.; Kim, P.; Winkler, R. Software solutions for evaluation and visualization of laser ablation inductively coupled plasma mass spectrometry imaging (LA-ICP-MSI) data: A short overview. J. Cheminform. 2019, 11, 16. [Google Scholar] [CrossRef]
- Allen Brain Reference Atlases. Available online: http://atlas.brain-map.org/ (accessed on 18 April 2019).
- Hare, D.J.; Kysenius, K.; Paul, B.; Knauer, B.; Hutchinson, R.W.; O’Connor, C.; Fryer, F.; Hennessey, T.P.; Bush, A.I.; Crouch, P.J.; et al. Imaging metals in brain tissue by laser ablation—Inductively coupled plasma—Mass spectrometry (LA-ICP-MS). J. Vis. Exp. 2017, 119. [Google Scholar] [CrossRef] [PubMed]
- Hare, D.J.; Raven, E.P.; Roberts, B.R.; Bogeski, M.; Portbury, S.D.; McLean, C.A.; Masters, C.L.; Connor, J.R.; Bush, A.I.; Crouch, P.J.; et al. Laser ablation-inductively coupled plasma-mass spectrometry imaging of white and gray matter iron distribution in Alzheimer’s disease frontal cortex. Neuroimage 2016, 137, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Brar, S.; Henderson, D.; Schenck, J.; Zimmerman, E.A. Iron accumulation in the substantia nigra of patients with Alzheimer disease and Parkinsonism. Arch. Neurol. 2009, 66, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Matusch, A.; Depboylu, C.; Palm, C.; Wu, B.; Höglinger, G.U.; Schäfer, M.K.; Becker, J.S. Cerebral bioimaging of Cu, Fe, Zn, and Mn in the MPTP mouse model of Parkinson’s disease using laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). J. Am. Soc. Mass Spectrom. 2010, 21, 161–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, J.S.; Matusch, A.; Palm, C.; Salber, D.; Morton, K.A.; Becker, J.S. Bioimaging of metals in brain tissue by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) and metallomics. Metallomics 2010, 2, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Pornwilard, M.-M.; Merle, U.; Weiskirchen, R.; Becker, J.S. Bioimiaging of copper deposition in Wilson’s disease mouse liver by laser ablation inductively coupled plasma mass spectrometry imaging (LA-ICP-MS). Int. J. Mass Spectrom. 2013, 354–355, 281–287. [Google Scholar] [CrossRef]
- Boaru, S.G.; Merle, U.; Uerlings, R.; Zimmermann, A.; Weiskirchen, S.; Matusch, A.; Stremmel, W.; Weiskirchen, R. Simultaneous monitoring of cerebral metal accumulation in an experimental model of Wilson’s disease by laser ablation inductively coupled plasma mass spectrometry. Bmc Neurosci. 2014, 15, 98. [Google Scholar] [CrossRef]
- Boaru, S.G.; Merle, U.; Uerlings, R.; Zimmermann, A.; Flechtenmacher, C.; Willheim, C.; Eder, E.; Ferenci, P.; Stemmel, W.; Weiskirchen, R. Laser ablation inductively coupled plasma mass spectrometry imaging of metals in experimental and clinical Wilson’s disease. J. Cell. Mol. Med. 2015, 19, 806–814. [Google Scholar] [CrossRef]
- Hachmöller, O.; Aichler, M.; Schwamborn, K.; Lutz, L.; Werner, M.; Sperling, M.; Walch, A.; Karst, U. Element bioimaging of liver needle biopsy specimens from patients with Wilson’s disease by laser ablation-inductively coupled plasma-mass spectrometry. J. Trace Elem. Med. Biol. 2016, 35, 97–102. [Google Scholar] [CrossRef]
- Hachmöller, O.; Zibert, A.; Zischka, H.; Sperling, M.; Groba, S.R.; Grünewald, I.; Wardelmann, E.; Schmidt, H.H.; Karst, U. Spatial investigation of the elemental distribution in Wilson’s disease liver after d-penicillamine treatment by LA-ICP-MS. J. Trace Elem. Med. Biol. 2017, 44, 26–31. [Google Scholar] [CrossRef]
- Hachmöller, O.; Aichler, M.; Schwamborn, K.; Lutz, L.; Werner, M.; Sperling, M.; Walch, A.; Karst, U. Investigating the influence of standard staining procedures on the copper distribution and concentration in Wilson’s disease liver samples by laser ablation-inductively coupled plasma-mass spectrometry. J. Trace Elem. Med. Biol. 2017, 44, 71–75. [Google Scholar] [CrossRef]
- Moreno, D.; Murillo, O.; Gazquez, C.; Hernández-Alcoceba, R.; Uerlings, R.; González-Aseguinolaza, G.; Weiskirchen, R. Visualization of the therapeutic efficacy of a gene correction approach in Wilson’s disease by laser-ablation inductively coupled mass spectrometry. J. Hepatol. 2018, 68, 1088–1090. [Google Scholar] [CrossRef]
- Uerlings, R.; Moreno, D.; Murillo, O.; Gazquez, C.; Hernández-Alcoceba, R.; González-Aseguinolaza, G.; Weiskirchen, R. Brain copper storage after genetic long-term correction in a mouse model of Wilson disease. Neurol. Genet. 2018, 4, e243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, J.C.; Lichtmannegger, J.; Zischka, H.; Sperling, M.; Karst, U. High spatial resolution LA-ICP-MS demonstrates massive liver copper depletion in Wilson disease rats upon Methanobactin treatment. J. Trace Elem. Med. Biol. 2018, 49, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Deng, J.; Borjigin, J. A new strain of rat for functional analysis of PINA. Brain Res. Mol. Brain Res. 2005, 137, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Buiakova, O.I.; Xu, J.; Lutsenko, S.; Zeitlin, S.; Das, K.; Das, S.; Ross, B.M.; Mekios, C.; Scheinberg, I.H.; Gilliam, T.C. Null mutation of the murine ATP7B (Wilson disease) gene results in intracellular copper accumulation and late-onset hepatic nodular transformation. Hum. Mol. Genet. 1999, 8, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Huster, D.; Purnat, T.D.; Burkhead, J.L.; Ralle, M.; Fiehn, O.; Stuckert, F.; Olson, N.E.; Teupser, D.; Lutsenko, S. High copper selectively alters lipid metabolism and cell cycle machinery in the mouse model of Wilson disease. J. Biol. Chem. 2007, 282, 8343–8355. [Google Scholar] [CrossRef] [PubMed]
- Murillo, O.; Luqui, D.M.; Gazquez, C.; Martinez-Espartosa, D.; Navarro-Blasco, I.; Monreal, J.I.; Guembe, L.; Moreno-Cermeno, A.; Corrales, F.J.; Prieto, J.; et al. Long-term metabolic correction of Wilson’s disease in a murine model by gene therapy. J. Hepatol. 2016, 64, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Ralle, M.; Huster, D.; Vogt, S.; Schirrmeister, W.; Burkhead, J.L.; Capps, T.R.; Gray, L.; Lai, B.; Maryon, E.; Lutsenko, S. Wilson disease at a single cell level: Intracellular copper trafficking activates compartment-specific responses in hepatocytes. J. Biol. Chem. 2010, 285, 30875–30883. [Google Scholar] [CrossRef]
- Vogt, S.; Ralle, M. Opportunities in multidimensional trace metal imaging: Taking copper-associated disease research to the next level. Anal. Bioanal. Chem. 2013, 405, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Weiskirchen, S.; Uerlings, R.; Kueppers, A.; Stellmacher, F.; Viveiros, A.; Zoller, H.; Weiskirchen, R. Quantification of liver iron overload disease with laser ablation inductively coupled plasma mass spectrometry. Bmc Med. Imaging 2018, 18, 51. [Google Scholar] [CrossRef]
- M-M, P.; Weiskirchen, R.; Gassler, N.; Bosserhoff, A.K.; Becker, J.S. Novel bioimaging techniques of metals by laser ablation inductively coupled plasma mass spectrometry for diagnosis of fibrotic and cirrhotic liver disorders. PLoS ONE 2013, 8, e58702. [Google Scholar] [CrossRef] [PubMed]
- Vašinová Galiová, M.; Copjaková, R.; Škoda, R.; Štepánková, K.; Vanková, M.; Kuta, J.; Prokeš, L.; Kynický, J.; Kanický, V. 2D elemental mapping of sections of human kidney stones using laser ablation inductively-coupled plasma-mass spectrometry: Possibilities and limitations. Spectrochim. Acta Part B Atom. Spectrosc. 2014, 100, 105–115. [Google Scholar] [CrossRef]
- Niedzwiecki, M.M.; Austin, C.; Remark, R.; Merad, M.; Gnjatic, S.; Estrada-Gutierrez, G.; Espejel-Nuñez, A.; Borboa-Olivares, H.; Guzman-Huerta, M.; Wright, R.J.; et al. A multimodal imaging workflow to visualize metal mixtures in the human placenta and explore colocalization with biological response markers. Metallomics 2016, 8, 444–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonta, M.; Gonzalez, J.J.; Derrick Quarles, C.; Russo, R.E.; Hegedus, B.; Limbeck, A. Elemental mapping of biological samples by the combined use of LIBS and LA-ICP-MS. J. Anal. Atom. Spectrom. 2016, 31, 252–258. [Google Scholar] [CrossRef]
- Queipo Abad, S.; Rodríguez-González, P.; García Alonso, J.I. Evidence of the direct adsorption of mercury in human hair during occupational exposure to mercury vapour. J. Trace Elem. Med. Biol. 2016, 36, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Su, X.; Xu, W.; Zhang, S.; Zhuo, X.; Ma, D. Determination of arsenic and lead in single hair strands by laser ablation inductively coupled plasma mass spectrometry. Sci. Rep. 2017, 7, 3426. [Google Scholar] [CrossRef] [Green Version]
- Eastman, R.R.; Jursa, T.P.; Benedetti, C.; Lucchini, R.G.; Smith, D.R. Hair as a biomarker of environmental manganese exposure. Environ. Sci. Technol. 2013, 47, 1629–1637. [Google Scholar] [CrossRef]
- Konz, I.; Fernández, B.; Fernández, M.L.; Pereiro, R.; González-Iglesias, H.; Coca-Prados, M.; Sanz-Medel, A. Quantitative bioimaging of trace elements in the human lens by LA-ICP-MS. Anal. Bioanal. Chem. 2014, 406, 2343–2348. [Google Scholar] [CrossRef]
- Roberts, B.R.; Doecke, J.D.; Rembach, A.; Yévenes, L.F.; Fowler, C.J.; McLean, C.A.; Lind, M.; Volitakis, I.; Masters, C.L.; Bush, A.I.; et al. AIBL research group. Rubidium and potassium levels are altered in Alzheimer’s disease brain and blood but not in cerebrospinal fluid. Acta Neuropathol. Commun. 2016, 4, 119. [Google Scholar] [CrossRef] [PubMed]
- Birka, M.; Wentker, K.S.; Lusmöller, E.; Arheilger, B.; Wehe, C.A.; Sperling, M.; Stadler, R.; Karst, U. Diagnosis of nephrogenic systemic fibrosis by means of elemental bioimaging and speciation analysis. Anal. Chem. 2015, 87, 3321–3328. [Google Scholar] [CrossRef]
- Doble, P.A.; Miklos, G.L.G. Distributions of manganese in diverse human cancers provide insights into tumour radioresistance. Metallomics 2018, 10, 1191–1210. [Google Scholar] [CrossRef]
- Aramendía, M.; Rello, L.; Bérail, S.; Donnard, A.; Pécheyran, C.; Resano, M. Direct analysis of dried blood spots by femtosecond-laser ablation-inductively coupled plasma-mass spectrometry. Feasibility of split-flow laser ablation for simultaneous trace element and isotopic analysis. J. Anal. At. Spectrom. 2015, 30, 296–309. [Google Scholar] [CrossRef] [Green Version]
- Nischkauer, W.; Vanhaecke, F.; Limbeck, A. Self-aliquoting micro-grooves in combination with laser ablation-ICP-mass spectrometry for the analysis of challenging liquids: Quantification of lead in whole blood. Anal. Bioanal. Chem. 2016, 408, 5671–5676. [Google Scholar] [CrossRef]
- Kröger, S.; Sperling, M.; Karst, U. Quantitative dried blood spot analysis for metallodrugs by laser ablation-inductively coupled plasma-mass spectrometry. J. Trace Elem. Med. Biol. 2019, 51, 50–56. [Google Scholar] [CrossRef]
- Chantada-Vázquez, M.P.; Moreda-Piñeiro, J.; Cantarero-Roldán, A.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Development of dried serum spot sampling techniques for the assessment of trace elements in serum samples by LA-ICP-MS. Talanta 2018, 186, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Lothian, A.; Lago, L.; Mukherjee, S.; Connor, A.R.; Fowler, C.; McLean, C.A.; Horne, M.; Masters, C.L.; Cappai, R.; Roberts, B.R. Characterization of the metal status of natively purified alpha-synuclein from human blood, brain tissue, or recombinant sources using size exclusion ICP-MS reveals no significant binding of Cu, Fe or Zn. Metallomics 2019, 11, 128–140. [Google Scholar] [CrossRef]
- Ferreira, C.R.; Gahl, W.A. Disorders of metal metabolism. Transl. Sci. Rare Dis. 2017, 2, 101–139. [Google Scholar] [CrossRef] [Green Version]
- Lauer, E.; Villa, M.; Jotterand, M.; Vilarino, R.; Bollmann, M.; Michaud, K.; Grabherr, S.; Augsburger, M.; Thomas, A. Imaging mass spectrometry of elements in forensic cases by LA-ICP-MS. Int. J. Leg. Med. 2017, 131, 497–500. [Google Scholar] [CrossRef]
- Weiskirchen, S.; Kim, P.; Weiskirchen, R. Determination of copper poisoning in Wilson’s disease using laser ablation inductively coupled plasma spectrometry. Ann. Transl. Med. 2018. [Google Scholar] [CrossRef]
- Uerlings, R.; Matusch, A.; Weiskirchen, R. Reconstruction of laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) spatial distribution images in Microsoft Excel 2007. Int. J. Mass Spectrom. 2016, 395, 27–35. [Google Scholar] [CrossRef]





| Specimens | Major Findings | Refs |
|---|---|---|
| Liver sections of Atp7b−/− and wild type mouse (each n = 5) | The average hepatic concentrations of Cu, Mn, Fe, and Zn were 4, 0.7, 41, and 18 µg/g tissue in control samples, while they were 143, 0.6, 80, and 32 µg/g tissue in livers of Atp7b−/− mouse | [37] |
| Brain sections of wild type (n = 8) and Atp7b−/− (n = 9) mice in the age range of 11-24 months | Cu was increased proportionally during ageing throughout all cerebral regions; Atp7b null mice showed ~2-fold stable increase of Cu throughout brain parenchyma; Cu was ~3.5-fold decreased in periventricular regions | [38] |
| Liver sections of wild type and Atp7b−/− mice and human WD liver samples | Liver sections of 10 months old Atp7b null mice and patients with WD showed irregular (patchy) Cu distribution with high regional concentrations; age-dependent accumulation of hepatic Cu was strictly associated with a simultaneous increase in iron (Fe) and Zn; human liver samples confirmed accumulation of hepatic Fe and Zn in WD patients; tumorigenic regions are highly enriched in Cu | [39] |
| Paraffin-embedded human liver needle biopsy (n = 2) | WD patients showed inhomogeneous Cu distribution and high Cu concentrations of up to 1200 μg/g; inverse correlation of regions with elevated Cu and region with high Fe concentrations | [40] |
| Liver of D-Penicillamine (DPA)-treated PINA/Atp7b−/− (LPP−/−)** rats and a liver samples from a patient before and after DPA treatment | DPA-treatment resulted in a significant decrease in hepatic Cu by more than a factor two; Cu distribution maps after DPA treatment were highly inhomogeneous and lowest Cu concentrations were found in direct proximity to blood vessels | [41] |
| Human stained and unstained liver needle biopsies (n = 8) | When comparing unstained and rhodanine-stained sections of each WD liver sample, unstained sections showed distinct structures of Cu distribution, while rhodanine-stained sections revealed blurred Cu distribution with 20–90% decreased concentrations | [42] |
| Liver sections of untreated or AAV-AAT-co-miATP7B-treated Atp7b−/− mice (n = 5) | While the mean of hepatic Cu was 112.7 ± 13.3 µg/g liver tissue in the untreated group, the delivery of the transgene reduced Cu content to a mean of 43.3 ± 3.6 µg/g liver tissue; removal of Cu provoked a simultaneous decrease in hepatic Fe (314 ± 38 vs. 150.2 ± 25.2 µg/g liver tissue) and a slight reduction in hepatic Zn (43.1 ± 3.5 vs. 32.4 ± 4.3 µg/g liver tissue) | [43] |
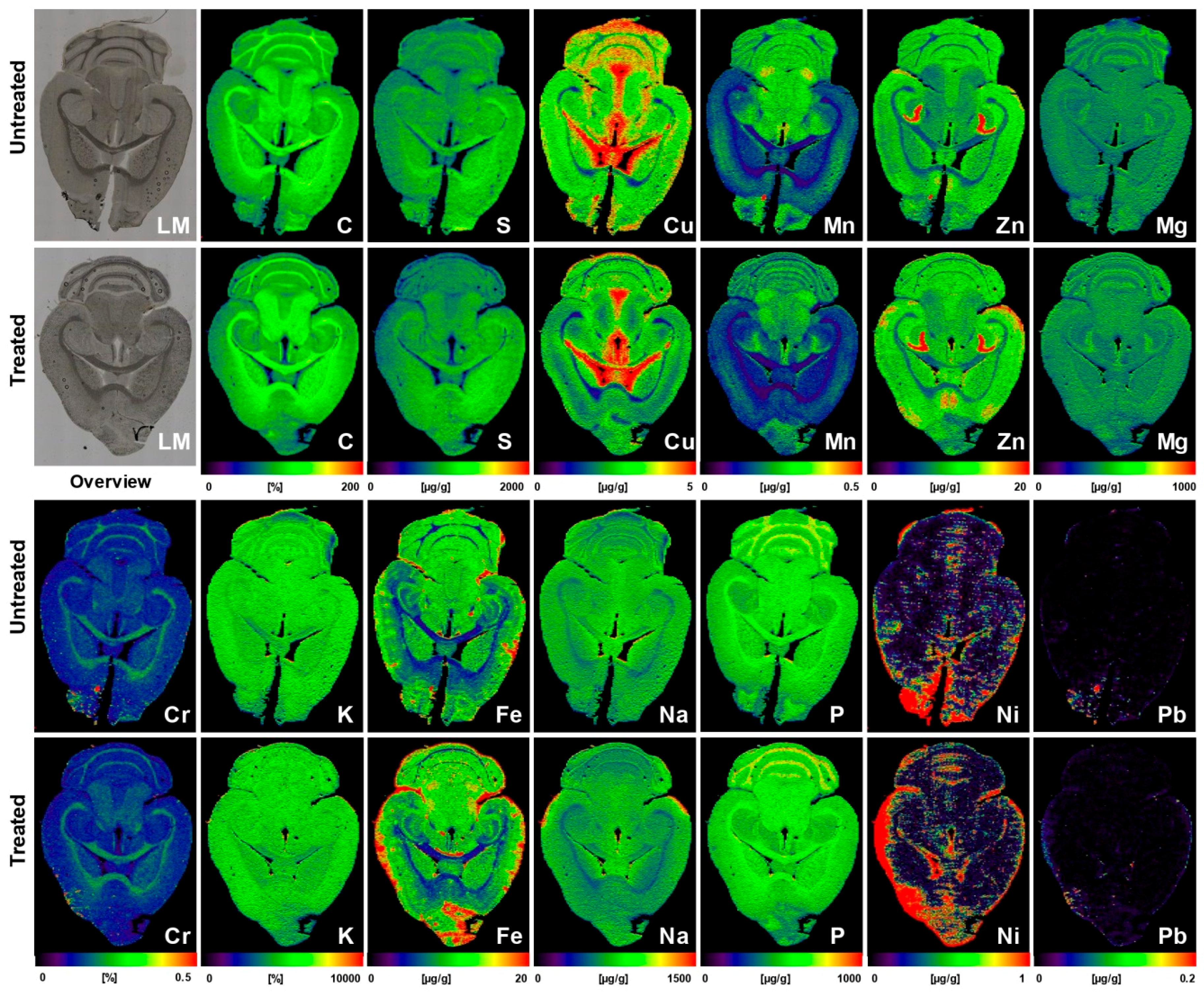
| Brain sections of untreated (n = 5) or AAV-AAT-co-miATP7B*-treated Atp7b−/− mice (n = 6) | Brains of animals receiving the transgene had overall lower concentrations of total cerebral Cu (3.8 ± 0.2 vs. 3.05 ± 0.17 µg/g brain tissue), most prominently noticeable in the cerebellum, cerebellar white matter, corpus callosum, 3rd and 4th ventricles, and surrounding tissue, and a slight decrease in the basal ganglia; the content in the Atp7b+/− control mice that showed no alterations in Cu metabolism was 2.34 ± 0.09 μg/g. Concentrations of Fe, Zn, Mn, Na, Mg, K, Ca, P, Cr, Ni, and Pb were unaffected by the therapeutic approach | [44] |
| Liver samples from PINA/Atp7b−/− (LPP−/−) rats treated with Methanobactin OB3b*** | Hepatic Cu hotspots were effectively removed by treatment with Methanobactin OB3b; Cu re-accumulation was observed after interruption of therapy | [45] |
| Parameter | Experimental Setting |
|---|---|
| ICP mass spectrometer | ICP-QMS (e.g., Thermo XSeries II*) |
| ICP RF power | 1450 W |
| Cooling gas flow rate | 16.0 L·min−1 |
| Auxiliary gas flow rate | 0.7 L·min−1 |
| Carrier gas flow rate | 1.0 L·min−1 |
| Dwell time | 20 ms |
| Extraction lens potential | 3400 V |
| Mass resolution (m/Δm) | 300 |
| Scanning mode | peak hopping |
| Typical analysis time per brain or liver sample (10 mm × 10 mm) | 4 h |
| Laser ablation system | New Wave (NRW213) |
| Wavelength of Nd:YAG** laser | 213 nm |
| Laser fluence | 0.24 J·cm−2 |
| Repetition frequency | 20 Hz |
| Laser spot size | 60–80 µm |
| Scan speed | 60 µm·s−1 |
| Ablation mode | line scan |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiskirchen, S.; Kim, P.; Weiskirchen, R. Laser Ablation Inductively Coupled Plasma Spectrometry: Metal Imaging in Experimental and Clinical Wilson Disease. Inorganics 2019, 7, 54. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7040054
Weiskirchen S, Kim P, Weiskirchen R. Laser Ablation Inductively Coupled Plasma Spectrometry: Metal Imaging in Experimental and Clinical Wilson Disease. Inorganics. 2019; 7(4):54. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7040054
Chicago/Turabian StyleWeiskirchen, Sabine, Philipp Kim, and Ralf Weiskirchen. 2019. "Laser Ablation Inductively Coupled Plasma Spectrometry: Metal Imaging in Experimental and Clinical Wilson Disease" Inorganics 7, no. 4: 54. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7040054