Photocracking Silica: Tuning the Plasmonic Photothermal Degradation of Mesoporous Silica Encapsulating Gold Nanoparticles for Cargo Release
Abstract
:1. Introduction
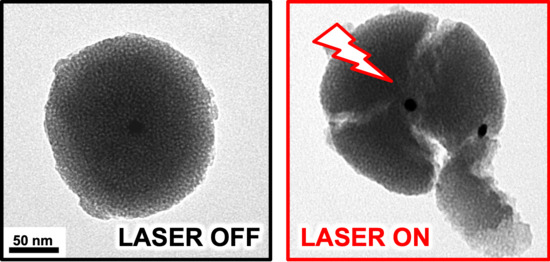
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Croissant, J.G.; Fatieiev, F.; Khashab, N.M. Degradability and clearance of silicon, organosilica, silsesquioxane, silica mixed oxide, and mesoporous silica nanoparticles. Adv. Mater. 2017, 29, 1604634. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Brinker, C.J. Biodegradable silica-based nanoparticles: Dissolution kinetics and selective bond cleavage. In The Enzymes; Elsevier: Amsterdam, The Netherlands, 2018; Volume 43, pp. 181–214. [Google Scholar]
- Croissant, J.; Cattoën, X.; Wong Chi Man, M.; Gallud, A.; Raehm, L.; Trens, P.; Maynadier, M.; Durand, J.O. Biodegradable ethylene-bis(propyl)disulfide-based periodic mesoporous organosilica nanorods and nanospheres for efficient in-vitro drug delivery. Adv. Mater. 2014, 26, 6174–6180. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Phua, S.Z.F.; Bindra, A.K.; Zhao, Y. Degradability and clearance of inorganic nanoparticles for biomedical applications. Adv. Mater. 2019, 31, 1805730. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hitzky, E.; Ariga, K.; Lvov, Y.M. Bio-Inorganic Hybrid Nanomaterials: Strategies, Synthesis, Characterization and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous silica and organosilica nanoparticles: Physical chemistry, biosafety, delivery strategies, and biomedical applications. Adv. Health. Mater. 2018, 7, 1700831. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Cattoën, X.; Wong Chi Man, M.; Durand, J.O.; Khashab, N.M. Syntheses and applications of periodic mesoporous organosilica nanoparticles. Nanoscale 2015, 7, 20318–20334. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.; Cattoën, X.; Wong Chi Man, M.; Dieudonné, P.; Charnay, C.; Raehm, L.; Durand, J.O. One-pot construction of multipodal hybrid periodic mesoporous organosilica nanoparticles with crystal-like architectures. Adv. Mater. 2015, 27, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Egger, S.M.; Hurley, K.R.; Datt, A.; Swindlehurst, G.; Haynes, C.L. Ultraporous mesostructured silica nanoparticles. Chem. Mater. 2015, 27, 3193–3196. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, L.L.; Jiang, J.G.; Calin, N.; Lam, K.F.; Zhang, S.J.; Wu, H.H.; Wu, G.D.; Albela, B.; Bonneviot, L.; et al. Facile large-scale synthesis of monodisperse mesoporous silica nanospheres with tunable pore structure. J. Am. Chem. Soc. 2013, 135, 2427–2430. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Y.; Wu, M.; Cai, X.; Yao, H.; Zhang, L.; Chen, H.; Shi, J. “Manganese extraction” strategy enables tumor-sensitive biodegradability and theranostics of nanoparticles. J. Am. Chem. Soc. 2016, 138, 9881–9894. [Google Scholar] [CrossRef]
- Hao, X.; Hu, X.; Zhang, C.; Chen, S.; Li, Z.; Yang, X.; Liu, H.; Jia, G.; Liu, D.; Ge, K.; et al. Hybrid mesoporous silica-based drug carrier nanostructures with improved degradability by hydroxyapatite. ACS Nano 2015, 9, 9614–9625. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Julfakyan, K.; Lu, J.; Emwas, A.H.; Anjum, D.H.; Omar, H.; Tamanoi, F.; Zink, J.I.; Khashab, N.M. Biodegradable oxamide-phenylene-based mesoporous organosilica nanoparticles with unprecedented drug payloads for delivery in cells. Chem. Eur. J. 2016, 22, 14806–14811. [Google Scholar] [CrossRef] [PubMed]
- Maggini, L.; Travaglini, L.; Cabrera, I.; Castro-Hartmann, P.; De Cola, L. Biodegradable peptide–silica nanodonuts. Chem. Eur. J. 2016, 22, 3697–3703. [Google Scholar] [CrossRef]
- Croissant, J.G.; Mauriello-Jimenez, C.; Maynadier, M.; Cattoën, X.; Wong Chi Man, M.; Raehm, L.; Mongin, O.; Blanchard-Desce, M.; Garcia, M.; Gary-Bobo, M.; et al. Synthesis of disulfide-based biodegradable bridged silsesquioxane nanoparticles for two-photon imaging and therapy of cancer cells. Chem. Commum. 2015, 51, 12324–12327. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Du, X.; Li, W.; Li, X.; Huang, H.; Liao, Q.; Shi, B.; Zhang, X.; Zhang, M. One-pot synthesis of redox-triggered biodegradable hybrid nanocapsules with a disulfide-bridged silsesquioxane framework for promising drug delivery. J. Mater. Chem. B 2017, 5, 4455–4469. [Google Scholar] [CrossRef]
- Moghaddam, S.P.H.; Yazdimamaghani, M.; Ghandehari, H. Glutathione-sensitive hollow mesoporous silica nanoparticles for controlled drug delivery. J. Control. Release 2018, 282, 62–75. [Google Scholar] [CrossRef]
- Du, X.; Kleitz, F.; Li, X.; Huang, H.; Zhang, X.; Qiao, S.Z. Disulfide-bridged organosilica frameworks: Designed, synthesis, redox-triggered biodegradation, and nanobiomedical applications. Adv. Funct. Mater. 2018, 28, 1707325. [Google Scholar] [CrossRef]
- Omar, H.; Croissant, J.G.; Alamoudi, K.; Alsaiari, S.; Alradwan, I.; Majrashi, M.A.; Anjum, D.H.; Martins, P.; Laamarti, R.; Eppinger, J.; et al. Biodegradable magnetic silica@iron oxide nanovectors with ultra-large mesopores for high protein loading, magnetothermal release, and delivery. J. Control. Release 2017, 259, 187–194. [Google Scholar] [CrossRef]
- Pohaku Mitchell, K.K.; Liberman, A.; Kummel, A.C.; Trogler, W.C. Iron(III)-doped, silica nanoshells: A biodegradable form of silica. J. Am. Chem. Soc. 2012, 134, 13997–14003. [Google Scholar] [CrossRef]
- Peng, Y.K.; Tseng, Y.J.; Liu, C.L.; Chou, S.W.; Chen, Y.W.; Tsang, S.E.; Chou, P.T. One-step synthesis of degradable T1-FeOOH functionalized hollow mesoporous silica nanocomposites from mesoporous silica spheres. Nanoscale 2015, 7, 2676–2687. [Google Scholar] [CrossRef]
- Wang, L.; Huo, M.; Chen, Y.; Shi, J. Iron-engineered mesoporous silica nanocatalyst with biodegradable and catalytic framework for tumor-specific therapy. Biomaterials 2018, 163, 1–13. [Google Scholar] [CrossRef]
- Fu, Y.; Jiang, Y.B.; Dunphy, D.; Xiong, H.; Coker, E.; Chou, S.; Zhang, H.; Vanegas, J.M.; Croissant, J.G.; Cecchi, J.L.; et al. Ultra-thin enzymatic liquid membrane for CO2 separation and capture. Nat. Commum. 2018, 9, 990. [Google Scholar] [CrossRef] [PubMed]
- De Vos, R.M.; Maier, W.F.; Verweij, H. Hydrophobic silica membranes for gas separation. J. Membr. Sci. 1999, 158, 277–288. [Google Scholar] [CrossRef]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-based mesoporous organic–inorganic hybrid materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef]
- Ma, Y.; Qi, L.; Ma, J.; Wu, Y.; Liu, O.; Cheng, H. Large-pore mesoporous silica spheres: Synthesis and application in HPLC. Colloids Surf. A 2003, 229, 1–8. [Google Scholar] [CrossRef]
- Heaney, P.J.; Prewitt, C.T.; Gibbs, G.V. (Eds.) Silica: Physical Behavior, Geochemistry, and Materials Applications; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2018; Volume 29. [Google Scholar]
- Margelefsky, E.L.; Zeidan, R.K.; Davis, M.E. Cooperative catalysis by silica-supported organic functional groups. Chem. Soc. Rev. 2008, 37, 1118–1126. [Google Scholar] [CrossRef] [Green Version]
- Croissant, J.G.; Zink, J.I.; Raehm, L.; Durand, J.O. Two-photon-excited silica and organosilica nanoparticles for spatiotemporal cancer treatment. Adv. Health. Mater. 2018, 7, 1701248. [Google Scholar] [CrossRef] [PubMed]
- Mauriello Jimenez, C.; Aggad, D.; Croissant, J.G.; Tresfield, K.; Laurencin, D.; Berthomieu, D.; Cubedo, N.; Rossel, M.; Alsaiari, S.; Anjum, D.H.; et al. Porous porphyrin-based organosilica nanoparticles for NIR two-photon photodynamic therapy and gene delivery in zebrafish. Adv. Funct. Mater. 2017, 28, 1800235. [Google Scholar] [CrossRef]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef]
- Rühle, B.; Saint-Cricq, P.; Zink, J.I. Externally controlled nanomachines on mesoporous silica nanoparticles for biomedical applications. ChemPhysChem 2016, 17, 1769–1779. [Google Scholar] [CrossRef]
- Dogra, P.; Adolphi, N.L.; Wang, Z.; Lin, Y.S.; Butler, K.S.; Durfee, P.N.; Croissant, J.G.; Noureddine, A.; Coker, E.N.; Bearer, E.L.; et al. Establishing the effects of mesoporous silica nanoparticle properties on in vivo disnmposition using imaging-based pharmacokinetics. Nat. Commum. 2018, 9, 4551. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, H.; Shi, J. In vivo bio-safety evaluations and diagnostic/therapeutic applications of chemically designed mesoporous silica nanoparticles. Adv. Mater. 2013, 25, 3144–3176. [Google Scholar] [CrossRef]
- Lee, D.; Beack, S.; Yoo, J.; Kim, S.K.; Lee, C.; Kwon, W.; Hahn, S.K.; Kim, C. In Vivo Photoacoustic Imaging of Livers Using Biodegradable Hyaluronic Acid-Conjugated Silica Nanoparticles. Adv. Mater. 2018, 28, 1800941. [Google Scholar] [CrossRef]
- Zhou, W.; Gao, X.; Liu, D.; Chen, X. Gold nanoparticles for in vitro diagnostics. Chem. Rev. 2015, 115, 10575–10636. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Marago, O.M.; Iati, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef]
- Daraee, H.; Eatemadi, A.; Abbasi, E.; Fekri Aval, S.; Kouhi, M.; Akbarzadeh, A. Application of gold nanoparticles in biomedical and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 410–422. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef]
- Stewart, M.E.; Anderton, C.R.; Thompson, L.B.; Maria, J.; Gray, S.K.; Rogers, J.A.; Nuzzo, R.G. Nanostructured plasmonic sensors. Chem. Rev. 2008, 108, 494–521. [Google Scholar] [CrossRef]
- Lu, X.; Rycenga, M.; Skrabalak, S.E.; Wiley, B.; Xia, Y. Chemical synthesis of novel plasmonic nanoparticles. Annu. Rev. Phys. Chem. 2009, 60, 167–192. [Google Scholar] [CrossRef]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors. Sens. Actuators B Chem. 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Liu, G.L.; Kim, J.; Lu, Y.; Lee, L.P. Optofluidic control using photothermal nanoparticles. Nat. Mater. 2006, 5, 27–32. [Google Scholar] [CrossRef]
- Rycenga, M.; Wang, Z.; Gordon, E.; Cobley, C.M.; Schwartz, A.G.; Lo, C.S.; Xia, Y. Probing the photothermal effect of gold-based nanocages with surface-enhanced Raman scattering (SERS). Angew. Chem. Int. Ed. 2009, 48, 9924–9927. [Google Scholar] [CrossRef]
- Raji, V.; Kumar, J.; Rejiya, C.S.; Vibin, M.; Shenoi, V.N.; Abraham, A. Selective photothermal efficiency of citrate capped gold nanoparticles for destruction of cancer cells. Exp. Cell Res. 2011, 317, 2052–2058. [Google Scholar] [CrossRef]
- Jones, M.R.; Millstone, J.E.; Giljohann, D.A.; Seferos, D.S.; Young, K.L.; Mirkin, C.A. Plasmonically controlled nucleic acid dehybridization with gold nanoprisms. ChemPhysChem 2009, 10, 1461–1465. [Google Scholar] [CrossRef]
- Zeng, N.; Murphy, A.B. Heat generation by optically and thermally interacting aggregates of gold nanoparticles under illumination. Nanotechnology 2009, 20, 375702. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV–Vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef]
- Hleb, E.Y.; Lapotko, D.O. Photothermal properties of gold nanoparticles under exposure to high optical energies. Nanotechnology 2008, 19, 355702. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, Z.; Lu, W.; Zhang, R.; Huang, Q.; Tian, M.; Li, L.; Liang, D.; Li, C. Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomaterials 2009, 30, 1928–1936. [Google Scholar] [CrossRef] [Green Version]
- Paasonen, L.; Laaksonen, T.; Johans, C.; Yliperttula, M.; Kontturi, K.S.; Urtti, A. Gold nanoparticles enable selective light-induced contents release from liposomes. J. Control. Release 2007, 122, 86–93. [Google Scholar] [CrossRef]
- Angelatos, A.S.; Radt, B.; Caruso, F. Light-responsive polyelectrolyte/gold nanoparticle microcapsules. J. Phys. Chem. B 2005, 109, 3071–3076. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Han, M.Y. Silica-coated metal nanoparticles. Chem. Asian J. 2010, 5, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Mikhailovsky, A.; Zasadzinski, J.A. Inside-out disruption of silica/gold core–shell nanoparticles by pulsed laser irradiation. Langmuir 2005, 21, 7528–7532. [Google Scholar] [CrossRef]
- Croissant, J.; Zink, J.I. Nanovalve-controlled cargo release activated by plasmonic heating. J. Am. Chem. Soc. 2012, 134, 7628–7631. [Google Scholar] [CrossRef] [PubMed]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Seeding growth for size control of 5–40 nm diameter gold nanoparticles. Langmuir 2001, 17, 6782–6786. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Enustun, B.V.; Turkevich, J. Coagulation of colloidal gold. J. Am. Chem. Soc. 1963, 85, 3317–3328. [Google Scholar] [CrossRef]
- Nozawa, K.; Gailhanou, H.; Raison, L.; Panizza, P.; Ushiki, H.; Sellier, E.; Delville, J.P.; Delville, M.H. Smart control of monodisperse Stöber silica particles: Effect of reactant addition rate on growth process. Langmuir 2005, 21, 1516–1523. [Google Scholar] [CrossRef]
- Mohamed, F.; Marchettini, P.; Stuart, O.A.; Urano, M.; Sugarbaker, P. Thermal enhancement of new chemotherapeutic agents at moderate hyperthermia. Ann. Surg. Oncol. 2003, 10, 463–468. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croissant, J.G.; Guardado-Alvarez, T.M. Photocracking Silica: Tuning the Plasmonic Photothermal Degradation of Mesoporous Silica Encapsulating Gold Nanoparticles for Cargo Release. Inorganics 2019, 7, 72. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7060072
Croissant JG, Guardado-Alvarez TM. Photocracking Silica: Tuning the Plasmonic Photothermal Degradation of Mesoporous Silica Encapsulating Gold Nanoparticles for Cargo Release. Inorganics. 2019; 7(6):72. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7060072
Chicago/Turabian StyleCroissant, Jonas G., and Tania M. Guardado-Alvarez. 2019. "Photocracking Silica: Tuning the Plasmonic Photothermal Degradation of Mesoporous Silica Encapsulating Gold Nanoparticles for Cargo Release" Inorganics 7, no. 6: 72. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7060072