Covalent Si–H Bonds in the Zintl Phase Hydride CaSiH1+x (x ≤ 1/3) †
Abstract
:1. Introduction
2. Results
2.1. The Crystal Structure of CaSiD1.1
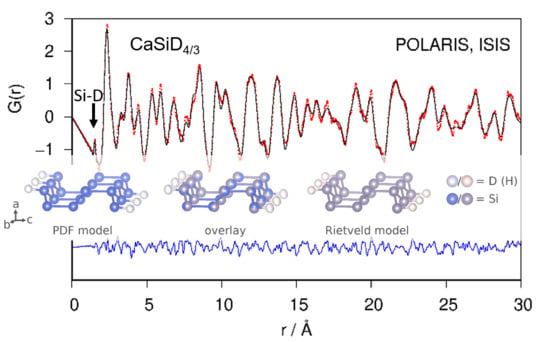
2.1.1. Total Scattering and PDF Analysis
2.1.2. Bragg Diffraction and Rietveld Analysis
2.2. In Situ Diffraction of the Hydrogenation Reaction and Hydrogen-Poor Phases
3. Discussion
3.1. The Crystal Structure of CaSiD1.1
3.2. Deuterium-Poor Phases
4. Conclusions
5. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gingl, F.; Vogt, T.; Akiba, E. Trigonal SrAl2H2: the first Zintl phase hydride. J. Alloys Compd. 2000, 306, 127–132. [Google Scholar] [CrossRef]
- Björling, T.; Noréus, D.; Häussermann, U. Polyanionic Hydrides from Polar Intermetallics AeE2 (Ae = Ca, Sr, Ba; E = Al, Ga, In). J. Am. Chem. Soc. 2006, 128, 817–824. [Google Scholar] [CrossRef]
- Lee, M.H.; Björling, T.; Hauback, B.C.; Utsumi, T.; Moser, D.; Bull, D.; Noréus, D.; Sankey, O.F.; Häussermann, U. Crystal structure, electronic structure, and vibrational properties of MAlSiH (M = Ca, Sr, Ba): Hydrogenation-induced semiconductors from the AlB2-type alloys MAlSi. Phys. Rev. B 2008, 78, 195209. [Google Scholar] [CrossRef]
- Lee, M.H.; Evans, M.J.; Daemen, L.L.; Sankey, O.F.; Häussermann, U. Vibrational Property Study of SrGa2H2 and BaGa2H2 by Inelastic Neutron Scattering and First Principles Calculations. Inorg. Chem. 2008, 47, 1496–1501. [Google Scholar] [CrossRef]
- Evans, M.J.; Holland, G.P.; Garcia-Garcia, F.J.; Häussermann, U. Polyanionic Gallium Hydrides from AlB2-Type Precursors AeGaE (Ae = Ca, Sr, Ba; E = Si, Ge, Sn). J. Am. Chem. Soc. 2008, 130, 12139–12147. [Google Scholar] [CrossRef] [PubMed]
- Kranak, V.F.; Evans, M.J.; Daemen, L.L.; Proffen, T.; Lee, M.H.; Sankey, O.F.; Häussermann, U. Structural and dynamic properties of the polyanionic hydrides SrAlGeH and BaAlGeH. Solid State Sci. 2009, 11, 1847–1853. [Google Scholar] [CrossRef]
- Evans, M.J.; Kranak, V.F.; Garcia-Garcia, F.J.; Holland, G.P.; Daemen, L.L.; Proffen, T.; Lee, M.H.; Sankey, O.F.; Häussermann, U. Structural and Dynamic Properties of BaInGeH: A Rare Solid-State Indium Hydride. Inorg. Chem. 2009, 48, 5602–5604. [Google Scholar] [CrossRef]
- Evans, M.J.; Lee, M.H.; Holland, G.P.; Daemen, L.L.; Sankey, O.F.; Häussermann, U. Vibrational properties of the gallium monohydrides SrGaGeH, BaGaSiH, BaGaGeH, and BaGaSnH. J. Solid State Chem. 2009, 182, 2068–2073. [Google Scholar] [CrossRef]
- Häussermann, U.; Kranak, V.F.; Puhakainen, K. Hydrogenous Zintl Phases: Interstitial Versus Polyanionic Hydrides. Struct. Bond. 2010, 139, 143–161. [Google Scholar] [CrossRef]
- Ångström, J.; Johansson, R.; Sarkar, T.; Sørby, M.H.; Zlotea, C.; Andersson, M.S.; Nordblad, P.; Scheicher, R.H.; Häussermann, U.; Sahlberg, M. Hydrogenation-Induced Structure and Property Changes in the Rare-Earth Metal Gallide NdGa: Evolution of a [GaH]2− Polyanion Containing Peierls-like Ga–H Chains. Inorg. Chem. 2016, 55, 345–352. [Google Scholar] [CrossRef]
- Auer, H.; Nedumkandathil, R.; Häussermann, U.; Kohlmann, H. The Hydrogenation of the Zintl Phase NdGa Studied by in situ Neutron Diffraction. Z. Anorg. Allg. Chem. 2019, 645, 175–181. [Google Scholar] [CrossRef]
- Nedumkandathil, R.; Kranak, V.F.; Johansson, R.; Ångström, J.; Balmes, O.; Andersson, M.S.; Nordblad, P.; Scheicher, R.H.; Sahlberg, M.; Häussermann, U. Hydrogenation Induced Structure and Property Changes in GdGa. J. Solid State Chem. 2016, 239, 184–191. [Google Scholar] [CrossRef]
- Werwein, A.; Benndorf, C.; Bertmer, M.; Franz, A.; Oeckler, O.; Kohlmann, H. Hydrogenation Properties of LnAl2 (Ln = La, Eu, Yb), LaGa2, LaSi2 and the Crystal Structure of LaGa2H0.71(2). Crystals 2019, 9, 193. [Google Scholar] [CrossRef]
- Aoki, M.; Ohba, N.; Noritake, T.; Towata, S. Reversible hydriding and dehydriding properties of CaSi: Potential of metal silicides for hydrogen storage. Appl. Phys. Lett. 2004, 85, 387–388. [Google Scholar] [CrossRef]
- Ohba, N.; Aoki, M.; Noritake, T.; Miwa, K.; Towata, S.-i. Development of New Hydrogen Storage Material CaSi Theoretical Prediction and Experiment. RD Rev. Toyota CRDL 2004, 39, 40–45. [Google Scholar]
- Aoki, M.; Ohba, N.; Noritake, T.; Towata, S.-i. Hydriding and dehydriding properties of CaSi. J. Alloys Compd. 2005, 404, 402–404. [Google Scholar] [CrossRef]
- Ohba, N.; Aoki, M.; Noritake, T.; Miwa, K.; Towata, S.-i. First-principles study of a hydrogen storage material CaSi. Phys. Rev. B 2005, 72, 075104. [Google Scholar] [CrossRef]
- Auer, H.; Guehne, R.; Bertmer, M.; Weber, S.; Wenderoth, P.; Hansen, T.C.; Haase, J.; Kohlmann, H. Hydrides of Alkaline Earth-Tetrel (AeTt) Zintl Phases: Covalent Tt–H Bonds from Silicon to Tin. Inorg. Chem. 2017, 56, 1061–1071. [Google Scholar] [CrossRef]
- Auer, H.; Schlegel, R.; Oeckler, O.; Kohlmann, H. Structural and Electronic Flexibility in Hydrides of Zintl Phases with Tetrel-Hydrogen and Tetrel-Tetrel Bonds. Angew. Chem. Int. Ed. 2017, 56, 12344–12347. [Google Scholar] [CrossRef] [Green Version]
- Auer, H.; Schlegel, R.; Oeckler, O.; Kohlmann, H. Strukturelle und elektronische Flexibilität in Hydriden von Zintl-Phasen mit Tetrel-Wasserstoff- und Tetrel-Tetrel-Bindung. Angew. Chem. 2017, 129, 12515–12518. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, W.; Udovic, T.J.; Rush, J.J.; Yildirim, T. Structure and hydrogen bonding in CaSiD1+x Issues about covalent bonding. Phys. Rev. B 2006, 74, 224101. [Google Scholar] [CrossRef]
- Armbruster, M.; Wörle, M.; Krumeich, F.; Nesper, R. Structure and Properties of Hydrogenated Ca, Sr, Ba, and Eu Silicides. Z. Anorg. Allg. Chem. 2009, 635, 1758–1766. [Google Scholar] [CrossRef]
- Guehne, R.; Auer, H.; Kohlmann, H.; Haase, J.; Bertmer, M. Determination of element-deuterium bond lengths in Zintl phase deuterides by 2H-NMR. Phys. Chem. Chem. Phys. 2019, 21, 10594–10602. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.W. Phenomenological model of anisotropic peak broadening in powder diffraction. J. Appl. Crystallogr. 1999, 32, 281–289. [Google Scholar] [CrossRef]
- Bruker AXS. TOPAS Version 5. Available online: www.bruker-axs.com (accessed on 25 June 2019).
- Auer, H.; Weber, S.; Hansen, T.C.; Többens, D.M.; Kohlmann, H. Reversible hydrogenation of the Zintl phases BaGe and BaSn studied by in situ diffraction. Z. Kristallogr. Cryst. Mater. 2018, 233, 399–409. [Google Scholar] [CrossRef] [Green Version]
- Auer, H.; Wallacher, D.; Hansen, T.C.; Kohlmann, H. In Situ Hydrogenation of the Zintl Phase SrGe. Inorg. Chem. 2017, 56, 1072–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anikina, E.Y.; Verbetsky, V.N. Investigation of hydrogen desorption from CaSiH by means of calorimetric method. J. Therm. Anal. Calorim. 2014, 118, 801–805. [Google Scholar] [CrossRef]
- Wenderoth, P. Untersuchungen zur Hydridbildung von Zintl-Phasen der Erdalkalimetalle mit Aluminium, Gallium und Silicium. Ph.D. Thesis, Universität des Saarlandes, Saarbrücken, Germany, 2014. [Google Scholar]
- Franz, A.; Hoser, A. E9: The Fine Resolution Powder Diffractometer (FIREPOD) at BER II. J. Large-Scale Res. Facil. 2017, 3, A103. [Google Scholar] [CrossRef]
- Hansen, T.C.; Henry, P.F.; Fischer, H.E.; Torregrossa, J.; Convert, P. The D20 instrument at the ILL: A versatile high-intensity two-axis neutron diffractometer. Meas. Sci. Technol. 2008, 19, 034001. [Google Scholar] [CrossRef]
- Kohlmann, H.; Kurtzemann, N.; Weihrich, R.; Hansen, T. In situ Neutron Powder Diffraction on Intermediate Hydrides of MgPd3 in a Novel Sapphire Gas Pressure Cell. Z. Anorg. Allg. Chem. 2009, 635, 2399–2405. [Google Scholar] [CrossRef]
- Götze, A.; Auer, H.; Finger, R.; Hansen, T.; Kohlmann, H. A sapphire single-crystal cell for in situ neutron powder diffraction of solid-gas reactions. Phys. B 2018, 551, 395–400. [Google Scholar] [CrossRef]
- Rietveld, H.M. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Crystallogr. 1967, 22, 151–152. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Hull, S.; Smith, R.I.; David, W.I.F.; Hannon, A.C.; Mayers, J.; Cywinski, R. The Polaris powder diffractometer at ISIS. Phys. B 1992, 180, 1000–1002. [Google Scholar] [CrossRef]
- Smith, R.I.; Hull, S.; Tucker, M.; Playford, H.Y.; McPhail, D.; Waller, S.; Norberg, S. The Upgraded Polaris Powder Diffractometer at ISIS. Rev. Sci. Inst. 2019. submitted for publication. [Google Scholar]
- Kohlmann, H.; Auer, H.; Playford, H.Y. Total scattering of Zintl phase deuterides CaSiD1.3, BaSiD1.9 and SrGeD1.3. STFC ISIS Neutron Muon Source 2018. [Google Scholar] [CrossRef]
- Soper, A.K. GudrunN and GudrunX programs for correcting raw neutron and X-ray diffraction data to differential scattering cross section, RAL Technical Report; Science and Technology Facilities Council: Oxforf, UK, 2011. [Google Scholar]
- Farrow, C.L.; Juhas, P.; Liu, J.W.; Bryndin, D.; Bozin, E.S.; Bloch, J.; Proffen, T.; Billinge, S.J.L. PDFfit2 and PDFgui: computer programs for studying nanostructure in crystals. J. Phys. Condens. Matter 2007, 19, 335219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GNUPLOT Version 5.0, Patchlevel 7. Available online: http://www.gnuplot.info (accessed on 25 June 2019).
- VESTA—Visualisation for Electronic and Structural Analysis. Version 3.3.1. Koichi Momma and Fujio Izumi: Ibaraki, Japan, 2018.
- Momma, K.; Izumi, F. VESTA3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]









| Rietveld | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Atom | x | y | z | Biso/Å2 | SOF | x | y | z | Biso/Å2 | SOF |
| Ca1 | 0.3381(3) | ¼ | 0.8453(5) | 1.07(18) | 1 | 0.3412 | ¼ | 0.8532 | 0.94 | 1 |
| Ca2 | 0.3113(2) | ¼ | 0.1571(5) | Biso(Ca1) | 1 | 0.3116 | ¼ | 0.1522 | 0.94 | 1 |
| Ca3 | 0.3592(2) | ¼ | 0.4810(4) | Biso(Ca1) | 1 | 0.3633 | ¼ | 0.4731 | 0.94 | 1 |
| Si1 | 0.0436(5) | ¼ | 0.1795(5) | 1.48(18) | 1 | 0.0349 | ¼ | 0.1795 | 1.03 | 1 |
| Si2 | 0.0430(5) | ¼ | 0.7608(5) | Biso(Si1) | 1 | 0.0430 | ¼ | 0.7640 | 1.03 | 1 |
| Si3 | 0.0417(4) | ¼ | 0.5270(6) | Biso(Si1) | 1 | 0.0396 | ¼ | 0.5373 | 1.03 | 1 |
| D1 | 0.2508(9) | ¼ | 0.8265(13) | 1.05(15) | 1 | 0.2436 | ¼ | 0.8408 | 1.26 | 1 |
| D2 | 0.2227(9) | ¼ | 0.1748(13) | Biso(D1) | 0.95(3) | 0.2219 | ¼ | 0.1699 | 1.26 | 1 |
| D3 | 0.2739(11) | ¼ | 0.4808(15) | Biso(D1) | 0.81(2) | 0.2816 | ¼ | 0.4853 | 1.26 | 0.89 |
| D4 | 0.0333(18) | ¼ | 0.045(2) | Biso(D1) | 0.468(15) | 0.0429 | ¼ | 0.0437 | 0.87 | 0.35 |
| Label | T/K | P/MPa | a/Å | b/Å | c/Å | SOF(D) | d(Si–Si)/Å |
|---|---|---|---|---|---|---|---|
| (a) | 524 | 5.5 | 4.5206(7) | 11.0166(14) | 3.9018(5) | 0.126(6) a | 2.519(9) |
| (b) | 535 | 3.0 | 4.5227(5) | 11.0069(10) | 3.9015(4) | 0.137(5) | 2.500(7) |
| (c) | 537 | 5.5 | 4.5184(5) | 11.0248(10) | 3.9012(3) | 0.168(6) | 2.492(7) |
| (d) | 529 | 0.2 | 4.5391(6) | 10.9521(15) | 3.9028(6) | 0.092(5) | 2.517(7) |
| (e) | 537 | 0.2 | 4.5406(6) | 10.9430(15) | 3.9036(6) | 0.096(6) | 2.520(9) |
| (f) | 537 | ≈10−5 | 4.5607(6) | 10.8469(12) | 3.9039(5) | 0.047(5) | 2.514(6) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auer, H.; Yang, F.; Playford, H.Y.; Hansen, T.C.; Franz, A.; Kohlmann, H. Covalent Si–H Bonds in the Zintl Phase Hydride CaSiH1+x (x ≤ 1/3). Inorganics 2019, 7, 106. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7090106
Auer H, Yang F, Playford HY, Hansen TC, Franz A, Kohlmann H. Covalent Si–H Bonds in the Zintl Phase Hydride CaSiH1+x (x ≤ 1/3). Inorganics. 2019; 7(9):106. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7090106
Chicago/Turabian StyleAuer, Henry, Fangshun Yang, Helen Y. Playford, Thomas C. Hansen, Alexandra Franz, and Holger Kohlmann. 2019. "Covalent Si–H Bonds in the Zintl Phase Hydride CaSiH1+x (x ≤ 1/3)" Inorganics 7, no. 9: 106. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7090106