Behavior of Compacted Magnesium-Based Powders for Energy-Storage Applications
Abstract
:1. Introduction
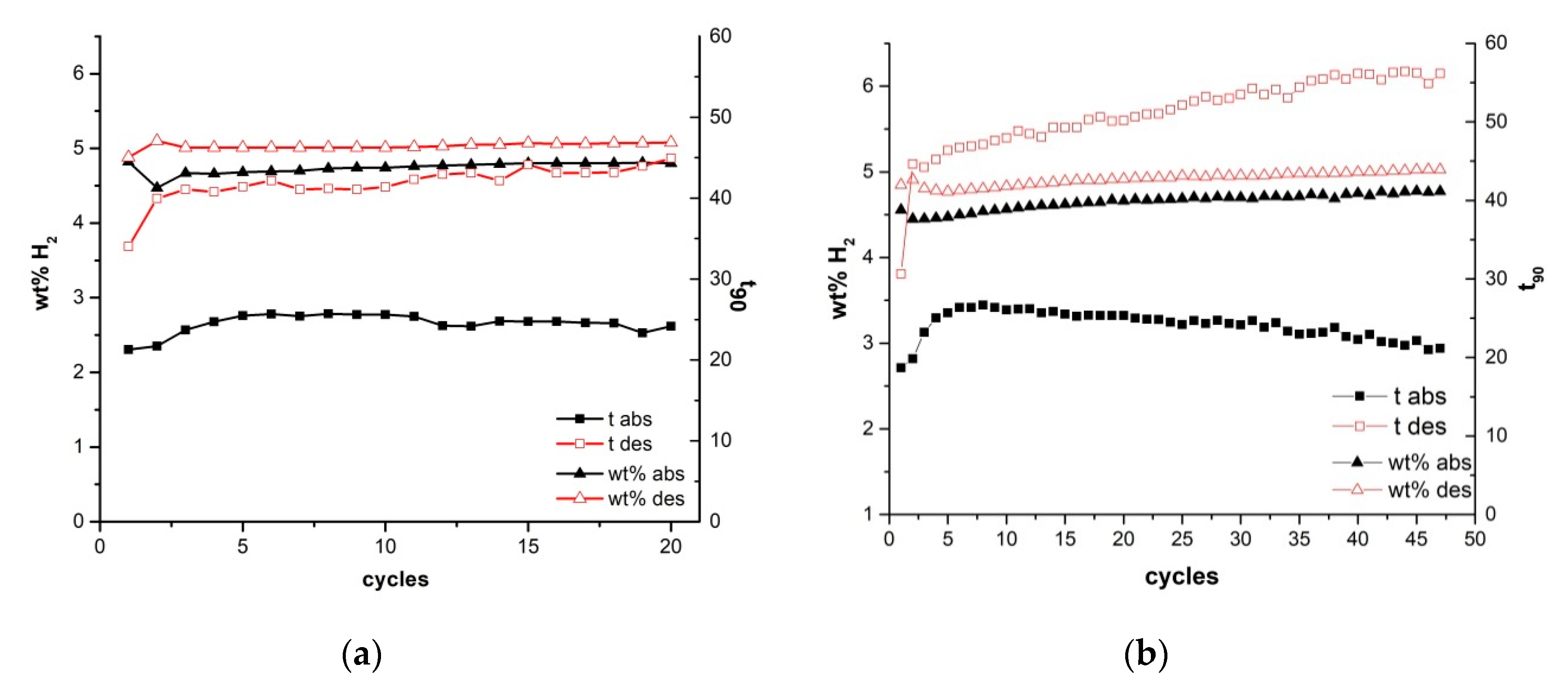
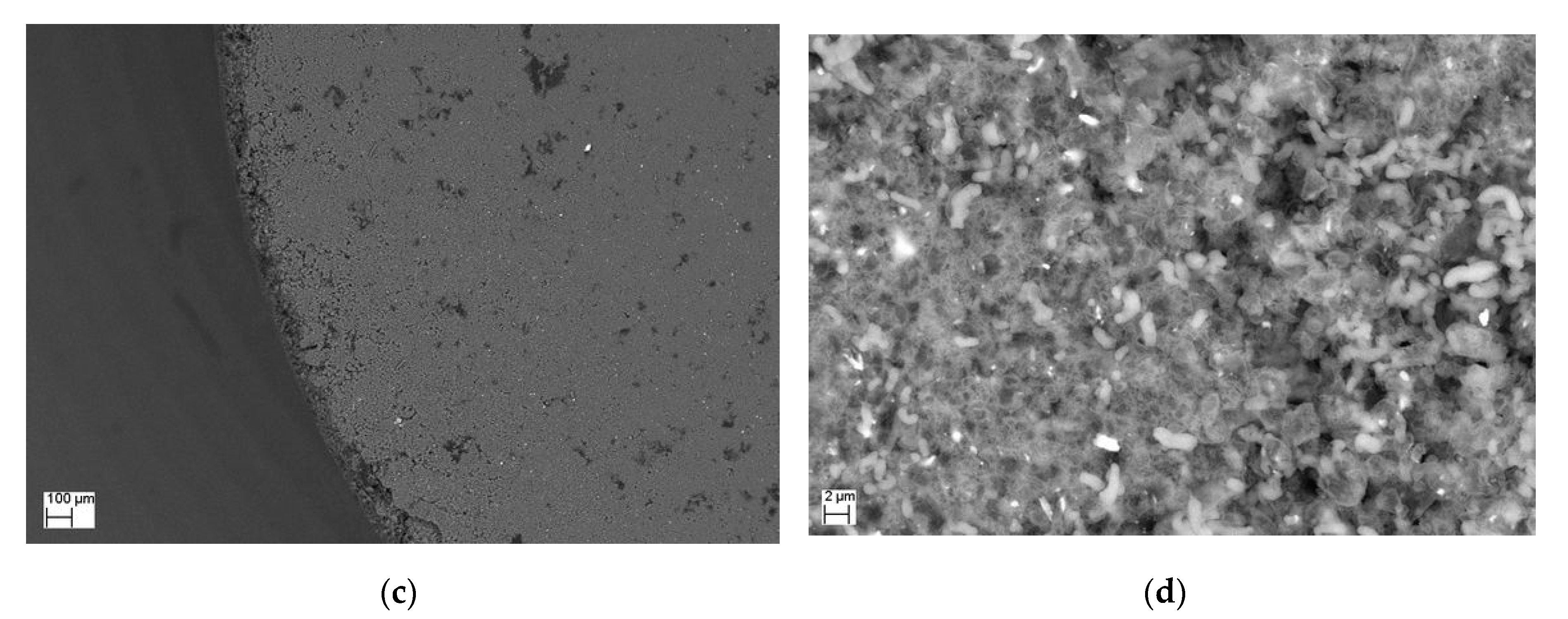
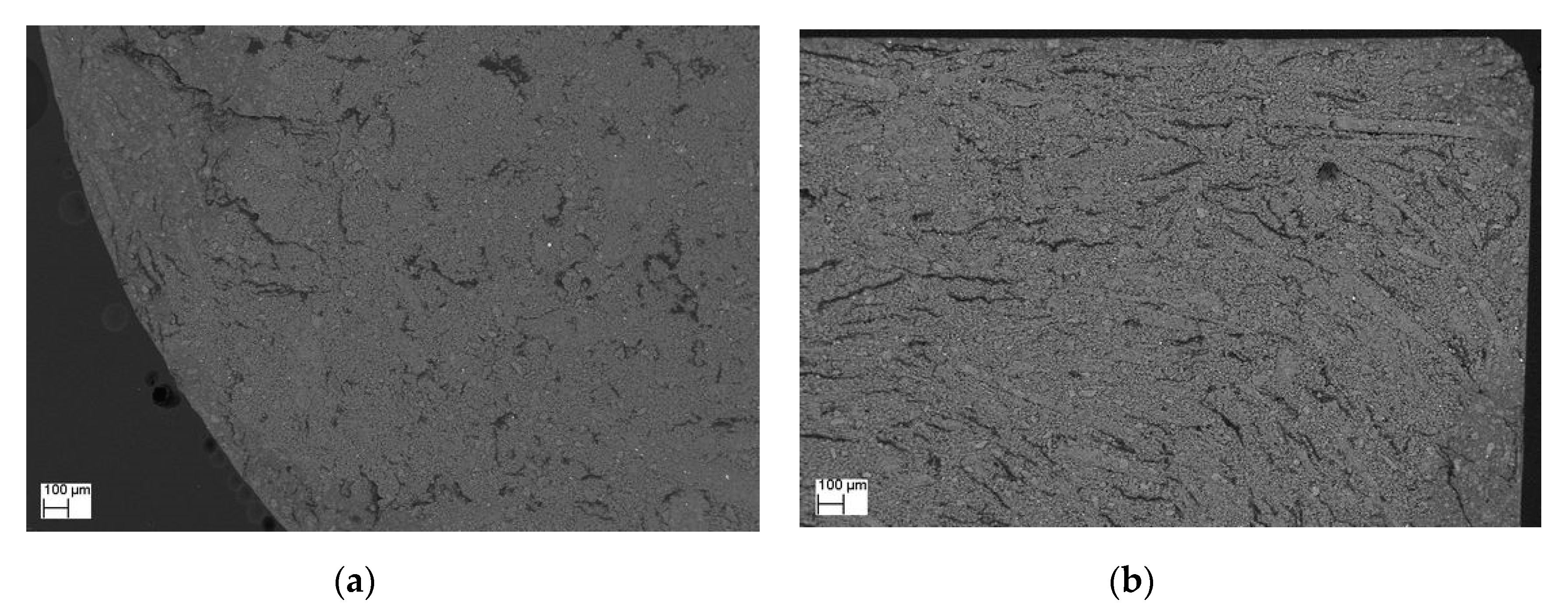
2. Results and Discussions
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moura, P.S.; de Almeida, A.T. The role of demand-side management in the grid integration of wind power. Appl. Energy 2010, 87, 2581–2588. [Google Scholar] [CrossRef]
- Ziemann, S.; Grunwald, A.; Schebek, L.; Müller, D.B.; Weil, M. The future of mobility and its critical raw materials. Rev. Métall. 2013, 110, 47–54. [Google Scholar] [CrossRef]
- Patricia, A.D.; Darina, B.; Claudiu, P.; Nikolaos, A. Cobalt: Demand-Supply Balances in the Transition to Electric Mobility; EUR 29381 EN; Publications Office of the European Union: Luxembourg, 2018; ISBN 978-92-79-94311-9. JRC112285. [Google Scholar] [CrossRef]
- Andersson, J.; Grönkvist, S. Large-scale storage of hydrogen. Int. J. Hydrogen Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Felderhoff, M.; Weidenthaler, C.; von Helmolt, R.; Eberle, U. Hydrogen storage: The remaining scientific and technological challenges. Phys. Chem. Chem. Phys. 2007, 9, 2643–2653. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef]
- Møller, K.T.; Jensen, T.R.; Akibab, E.; Li, H.-W. Hydrogen—A sustainable energy carrier. Prog. Nat. Sci. Mater. Int. 2017, 27, 34–40. [Google Scholar] [CrossRef]
- Makepeace, J.W.; He, T.; Weidenthaler, C.; Jensen, T.R.; Chang, F.; Vegge, T.; Ngene, P.; Kojima, Y.; de Jongh, P.E.; Chen, P. Reversible ammonia-based and liquid organic hydrogen carriers for high density hydrogen storage: Recent progress. Int. J. Hydrogen Energy 2019, 44, 7746–7767. [Google Scholar] [CrossRef]
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid organic hydrogen carriers (LOHCs): Toward a hydrogen-free hydrogen economy. Acc. Chem. Res. 2017, 50, 74–85. [Google Scholar] [CrossRef]
- Aakko-Saksa, P.T.; Cook, C.; Kiviaho, J.; Repo, T. Liquid organic hydrogen carriers for transportation and storing of renewable energy—Review and discussion. J. Power Sources 2018, 396, 803–823. [Google Scholar] [CrossRef]
- Yoo, J.S.; Christensen, R.; Vegge, T.; Nørskov, J.K.; Studt, F. Theoretical insight into the trends that guide the electrochemical reduction of carbon dioxide to formic acid. ChemSusChem 2016, 9, 358–363. [Google Scholar] [CrossRef]
- ACIL Allen Consulting, Opportunities for Australia from Hydrogen Exports, ACIL Allen Consulting for ARENA, 2018. Available online: https://arena.gov.au/assets/2018/08/opportunities-for-australia-from-hydrogenexports.pdf (accessed on 20 May 2020).
- International Energy Agency. The Future of Hydrogen: Seizing Today’s Opportunities, 2019. Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 20 May 2020).
- Hirscher, M.; Yartys, V.A.; Baricco, M.; von Colbe, J.B.; Blanchard, D.; Bowman, R.C., Jr.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for hydrogen-based energy storage-past, recent progress and future outlook. J. Alloys Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Züttel, A.; Rentsch, S.; Fischer, P.; Wenger, P.; Sudan, P.; Mauron, P.; Emmenegger, C. Hydrogen storage properties of LiBH4. J. Alloys Compd. 2003, 356–357, 515–520. [Google Scholar] [CrossRef]
- Milanese, C.; Jensen, T.R.; Hauback, B.C.; Pistidda, C.; Dornheim, M.; Yang, H.; Lombardo, L.; Züttel, A.; Filinchuk, Y.; Ngene, P.; et al. Complex hydrides for energy storage. Int. J. Hydrogen Energy 2019, 44, 7860–7874. [Google Scholar] [CrossRef] [Green Version]
- Callini, E.; Atakli, Z.O.K.; Hauback, B.C.; Orimo, S.-I.; Jensen, C.; Dornheim, M.; Grant, D.; Cho, Y.W.; Chen, P.; Hjörvarsson, B.; et al. Complex and liquid hydrides for energy storage. Appl. Phys. A 2016, 122, 353. [Google Scholar] [CrossRef]
- Ley, B.M.; Jepsen, L.H.; Lee, Y.-S.; Cho, Y.W.; Von Colbe, J.M.B.; Dornheim, M.; Rokni, M.; Jensen, J.O.; Sloth, M.; Filinchuk, Y.; et al. Complex hydrides for hydrogen storage—New perspectives. Mater. Today 2014, 17, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Mauron, P.; Buchter, F.; Friedrichs, O.; Remhof, A.; Bielmann, M.; Zwicky, C.N.; Züttel, A. Stability and Reversibility of LiBH4. J. Phys. Chem. B 2008, 112, 906–910. [Google Scholar] [CrossRef]
- Møller, K.T.; Sheppard, D.; Ravnsbæk, D.B.; Buckley, C.E.; Akiba, E.; Li, H.-W.; Jensen, T.R. Complex Metal Hydrides for Hydrogen, Thermal and Electrochemical Energy Storage. Energies 2017, 10, 1645. [Google Scholar] [CrossRef] [Green Version]
- Paskevicius, M.; Jepsen, L.H.; Schouwink, P.; Cerny, R.; Ravnsbaek, D.B.; Filinchuk, Y.; Dornheim, M.; Besenbacher, F.; Jensen, T.R. Metal borohydrides and derivatives—Synthesis, structure and properties. Chem. Soc. Rev. 2017, 46, 1565. [Google Scholar] [CrossRef]
- Jain, I.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrogen Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Dornheim, M.; Doppiu, S.; Barkhordarian, G.; Boesenberg, U.; Klassen, T.; Gutfleisch, O.; Bormann, R. Hydrogen storage in Mg based hydrides and hydride composites. Scr. Mater. 2007, 56, 841–846. [Google Scholar] [CrossRef]
- Crivello, J.-C.; Denys, R.V.; Dornheim, M.; Felderhoff, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Walker, G.S.; et al. Mg-based compounds for hydrogen and energy storage. Appl. Phys. A 2016, 122, 85. [Google Scholar] [CrossRef] [Green Version]
- Crivello, J.-C.; Dam, B.; Denys, R.V.; Dornheim, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Milanese, C.; et al. Review of magnesium hydride-based materials: Development and optimization. Appl. Phys. A 2016, 122, 97. [Google Scholar] [CrossRef] [Green Version]
- Yartis, V.A.; Lototskyy, M.V.; Akiba, E.; Albert, R.; Antonov, V.E.; Ares, J.R.; Baricco, M.; Bourgeois, N.; Buckley, C.E.; von Colbe, J.M.B.; et al. Magnesium based materials for hydrogen based energy storage: Past, present and future. Int. J. Hydrogen Energy 2019, 44, 7809–7859. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, C.; Lai, Q.; Liu, W.; Wang, D.-W.; Aguey-Zinsou, K.-F. Tailoring magnesium based materials for hydrogen storage through synthesis: Current state of the art. Energy Storage Mater. 2018, 10, 168–198. [Google Scholar] [CrossRef]
- Paskevicius, M.; Sheppard, D.A.; Williamson, K.; Buckley, C.E. Metal hydride thermal heat storage prototype for concentrating solar thermal power. Energy 2015, 88, 469–477. [Google Scholar] [CrossRef]
- Urbanczyk, R.; Peinecke, K.; Peil, S.; Felderhoff, M. Development of a heat storage demonstration unit on the basis of Mg2FeH6 as heat storage material and molten salt as heat transfer media. Int. J Hydrogen Energy 2017, 42, 13818–13826. [Google Scholar] [CrossRef]
- Urbanczyk, R.; Meggouh, M.; Moury, R.; Peinecke, K.; Peil, S.; Felderhoff, M. Demonstration of Mg2FeH6 as heat storage material at temperatures up to 550 °C. Appl. Phys. A 2016, 122, 315. [Google Scholar] [CrossRef] [Green Version]
- Bogdanovič, B.; Hartwing, T.H.; Spliethoff, B. The development, testing and optimization of energy storage materials based on the MgH2-Mg system. Int. J. Hydrogen Energy 1993, 18, 575–589. [Google Scholar] [CrossRef]
- Yuan, H.; An, Y.; Xu, G.; Chen, C. Hydriding behavior of magnesium-based hydrogen storage alloy modified by mechanical ball-milling. Mater. Chem. Phys. 2004, 83, 340–344. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Manickam, K.; Hirscher, M.; Besenbacher, F.; Jensen, T.R. Confinement of MgH2 nanoclusters within nanoporous aerogel scaffold materials. ACS Nano 2009, 3, 3521–3528. [Google Scholar] [CrossRef]
- De Jongh, P.E.; Adelhelm, P. Nanosizing and Nanoconfinement: New Strategies towards Meeting Hydrogen Storage Goals. ChemSusChem 2010, 3, 1332–1348. [Google Scholar] [CrossRef] [PubMed]
- Huen, P.; Paskevicius, M.; Richter, B.; Ravnsbaek, D.B.; Jensen, T.R. Hydrogen Storage Stability of Nanoconfined MgH2 upon Cycling. Inorganics 2017, 5, 57. [Google Scholar] [CrossRef]
- Huot, J.; Tremblay, M.L.; Schulz, R. Synthesis of nanocrystalline hydrogen storage materials. J. Alloys Compd. 2003, 356–357, 603–607. [Google Scholar] [CrossRef]
- Alsabawi, K.; Webb, T.A.; Gray, E.M.; Webb, C.J. Effect of C60 Additive on Magnesium Hydride for Hydrogen Storage. Int. J. Hydrogen Energy 2015, 40, 10508–10515. [Google Scholar] [CrossRef]
- Bouaricha, S.; Dodelet, J.P.; Guay, D.; Huot, J.; Schulz, R. Study of the activation process of Mg-based hydrogen storage materials modified by graphite and other carbonaceous compounds. J. Mater. Res. 2011, 16, 2893–2905. [Google Scholar] [CrossRef]
- de Rango, P.; Marty, P.; Fruchart, D. Hydrogen storage systems based on magnesium hydride: From laboratory tests to fuel cell integration. Appl. Phys. A 2016, 122, 126. [Google Scholar] [CrossRef]
- Webb, C.J. A review of catalyst-enhanced magnesium hydride as a hydrogen storage material. J. Phys. Chem. Solids 2015, 84, 96–106. [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.-F.; Nicolaisen, T.; Fernandez, A.J.R.; Klassen, T.; Bormann, R. Effect of nanosized oxides on MgH2 (de)hydriding kinetics. J. Alloys Compd. 2007, 434–435, 738–742. [Google Scholar] [CrossRef]
- Bhat, V.V.; Rougier, A.; Aymarda, L.; Darok, X.; Nazri, G.; Tarascon, J.M. Catalytic activity of oxides and halides on hydrogen storage of MgH2. J. Power Sources 2006, 159, 107–110. [Google Scholar] [CrossRef]
- Bogdanovič, B.; Reiser, A.; Schlichte, K.; Spliethoff, B.; Tesche, B. Thermodynamics and dynamics of the Mg-Fe-H system and its potential for thermochemical thermal energy storage. J. Alloys Compd. 2002, 345, 77–89. [Google Scholar] [CrossRef]
- Miwa, K.; Takagi, S.; Matsuo, M.; Orimo, S.-I. Thermodynamical stability of complex transition metal hydrides Mg2FeH6. J. Phys. Chem. C 2013, 117, 8014–8019. [Google Scholar] [CrossRef]
- Norek, M.; Nielsen, T.K.; Polanski, M.; Kunce, I.; Plocinski, T.; Jaroszewicz, L.R.; Cerenius, Y.; Jensen, T.R.; Bystrzycki, J. Synthesis and decomposition mechanisms of ternary Mg2CoH5 studied using in situ synchrotron X-ray diffraction. Int. J. Hydrogen Energy 2011, 36, 10760–10770. [Google Scholar] [CrossRef]
- Reilly, J.J.; Wiswall, R.H. Reaction of hydrogen with alloys of magnesium and nickel and the formation of Mg2NiH4. Inorg. Chem. 1968, 7, 2254–2256. [Google Scholar] [CrossRef]
- Polanski, M.; Nielsen, T.K.; Kunce, I.; Norek, M.; Płociński, T.; Jaroszewicz, L.R.; Gundlach, C.; Jensen, T.R.; Bystrzyckia, J. Mg2NiH4 synthesis and decomposition reactions. Int. J. Hydrogen Energy 2013, 38, 4003–4010. [Google Scholar] [CrossRef]
- Di Chio, M.; Ziggiotti, A.; Baricco, M. Effect of microstructure on hydrogen absorption in LaMg2Ni. Intermetallics 2008, 16, 102–106. [Google Scholar] [CrossRef]
- Huang, B.; Yvon, K.; Fischer, P. New Quaternary metal hydrides with CaMgNiH4-type structure. J. Alloys Compd. 1994, 204, L5–L8. [Google Scholar] [CrossRef]
- Huang, B.; Yvon, K.; Fischer, P. Calcium magnesium nickel(0) tetrahydride, CaMgNiH4, containing tetrahedral [NiH4]4− complex anions: The first quaternary transition metal hydride. J. Alloys Compd. 1992, 178, 173–179. [Google Scholar] [CrossRef]
- Humphries, T.D.; Takagi, S.; Li, G.; Matsuo, M.; Sato, T.; Sørby, M.H.; Deledda, S.; Hauback, B.C.; Orimo, S.-I. Complex transition metal hydrides incorporating ionic hydrogen: Synthesis and characterization of Na2Mg2FeH8 and Na2Mg2RuH8. J. Alloys Compd. 2015, 645, S347–S352. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Yvon, K.; Fischer, P. Synthesis, structure and thermal stability of Yb4Mg4Fe3H2. J. Alloys Compd. 1993, 197, 65–68. [Google Scholar] [CrossRef]
- Bobet, J.L.; Krawiec, S.D.; Grigorova, E.; Cansell, R.; Chevalier, B. Addition of nanosized Cr2O3 to magnesium for improvement of the hydrogen sorption properties. J. Alloys Compd. 2003, 351, 217. [Google Scholar] [CrossRef]
- Huot, J.; Cuevas, F.; Deledda, S.; Edalati, K.; Filinchuk, Y.; Grosdidier, T.; Hauback, B.C.; Heere, M.; Jensen, T.R.; Latroche, M.; et al. Mechanochemistry of Metal Hydrides: Recent Advances. Materials 2019, 12, 2778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneemann, A. Nanostructured Metal Hydrides for Hydrogen Storage. Chem. Rev. 2018, 118, 10775–10839. [Google Scholar] [CrossRef] [PubMed]
- Hanada, N.; Ichikawa, T.; Fujii, H. Catalytic Effect of Nanoparticle 3d-Transition Metals on Hydrogen Storage Properties in Magnesium Hydride MgH2 Prepared by Mechanical Milling. J. Phys. Chem. B 2005, 109, 7188–7194. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. Catalytic effect of transition metals on hydrogen sorption in nanocrystalline ball milled MgH2–Tm (Tm = Ti, V, Mn, Fe and Ni) systems. J. Alloys Compd. 1999, 292, 247–252. [Google Scholar] [CrossRef]
- Bassetti, A.; Bonetti, E.; Pasquini, L.; Montone, A.; Grbovic, J.; Antisari, M.V. Hydrogen desorption from ball milled MgH2 catalyzed with Fe. Eur. Phys. J. B 2005, 43, 19–27. [Google Scholar] [CrossRef]
- Shang, C.X.; Bououdina, M.; Song, Y.; Guo, Z.X. Mechanical alloying and electronic simulations of (MgH2+M) systems (M = Al, Ti, Fe, Ni, Cu and Nb) for hydrogen storage. Int. J. Hydrogen Energy 2004, 29, 73–80. [Google Scholar] [CrossRef]
- Cabo, M.; Garroni, S.; Pellicer, E.; Milanese, C.; Girella, A.; Marini, A.; Rossinyol, E.; Suriñach, S.; Baró, M.D. Hydrogen sorption performance of MgH2 doped with mesoporous nickel- and cobalt-based oxides. Int. J. Hydrogen Energy 2011, 36, 5400–5410. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Fast hydrogen sorption kinetics of nanocrystalline Mg using Nb2O5 as catalyst. Scr. Mater. 2003, 49, 213–217. [Google Scholar] [CrossRef]
- Rahman, M.W.; Castellero, A.; Enzo, S.; Livraghi, S.; Giamello, E.; Baricco, M. Effect of Mg–Nb oxides addition on hydrogen sorption in MgH2. J. Alloys Compd. 2011, 509, S438–S443. [Google Scholar] [CrossRef]
- Montone, A.; Aurora, A.; Mirabile Gattia, D.; Antisari, M.V. Microstructural and kinetic evolution of Fe doped MgH2 during H2 cycling. Catalysts 2012, 2, 400–411. [Google Scholar] [CrossRef] [Green Version]
- Mirabile Gattia, D.; Jangir, M.; Jain, I.P. Study on nanostructured MgH2 with Fe and its oxides for hydrogen storage applications. J. Alloys Compd. 2019, 801, 188–191. [Google Scholar] [CrossRef]
- Polanski, M.; Nielsen, T.K.; Cerenius, Y.; Bystrzycki, J.; Jensen, T.R. Synthesis and decomposition mechanisms of Mg2FeH6 studied by in-situ synchrotron X-ray diffraction and high-pressure DSC. Int. J. Hydrogen Energy 2010, 35, 3578–3582. [Google Scholar] [CrossRef]
- Puszkiel, J.; Gennari, F.; Larochette, P.A.; Karimi, F.; Pistidda, C.; Utke, G.R.; Jepsen, J.; Jensen, T.R.; Gundlach, C.; von Colbe, J.B. Sorption behavior of the MgH2–Mg2FeH6 hydride storage system synthesized by mechanical milling followed by sintering. Int. J. Hydrogen Energy 2013, 38, 14618–14630. [Google Scholar] [CrossRef]
- Study on the Review of the List of Critical Raw Materials, Criticality Assessments, Final Report, European Commission 2017. Available online: https://op.europa.eu/en/publication-detail/-/publication/08fdab5f-9766-11e7-b92d-01aa75ed71a1 (accessed on 20 May 2020).
- Pistidda, C.; Bergemann, N.; Wurr, J.; Rzeszutek, A.; Moller, K.T.; Hansen, B.R.S.; Garroni, S.; Horstmann, C.; Milanese, C.; Girella, A.; et al. Hydrogen storage systems from waste Mg alloys. J. Power Sources 2014, 270, 554–563. [Google Scholar] [CrossRef]
- Hardian, R.; Pistidda, C.; Chaudhary, A.-L.; Capurso, G.; Gizer, G.; Cao, H.; Milanese, C.; Girella, A.; Santoru, A.; Yigit, D.; et al. Waste Mg-Al based alloys for hydrogen storage. Int. J. Hydrogen Energy 2018, 43, 16738–16748. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Ali, N.; Al-Salem, S.M. Solid-State conversion of magnesium waste to advanced hydrogen-storage nanopowder particles. Nanomaterials 2020, 10, 1037. [Google Scholar] [CrossRef]
- Kim, K.J.; Montoya, B.; Razani, A.; Lee, K.H. Metal hydride compacts of improved thermal conductivity. Int. J. Hydrogen Energy 2001, 26, 609–613. [Google Scholar] [CrossRef]
- Eaton, E.; Olsen, C.; Sheinberg, H.; Steyert, W. Mechanically stable hydride composites designed for rapid cycling. Int. J. Hydrogen Energy 1981, 6, 609–623. [Google Scholar] [CrossRef]
- Ishikawa, H.; Oguro, K.; Kato, A.; Suzuki, H.; Ishii, E. Preparation and properties of hydrogen storage alloy-copper microcapsules. J. Less Common Met 1985, 107, 105–110. [Google Scholar] [CrossRef]
- Khandelwal, A.; Agresti, F.; Capurso, G.; Russo, S.; Maddalena, A.; Gialanella, S.; Principi, G. Pellets of MgH2-based composites as practical material for solid state hydrogen storage. Int. J. Hydrogen Energy 2010, 35, 3565–3571. [Google Scholar] [CrossRef] [Green Version]
- Pohlmann, C.; Rontzsch, L.; Kalinichenka, S.; Hutsch, T.; Kieback, B. Magnesium alloy-graphite composites with tailored heat conduction properties for hydrogen storage applications. Int. J. Hydrogen Energy 2010, 35, 12829–12836. [Google Scholar] [CrossRef]
- Klein, H.P.; Groll, M. Heat transfer characteristics of expanded graphite matrices in metal hydride beds. Int. J. Hydrogen Energy 2004, 29, 1503–1511. [Google Scholar] [CrossRef]
- Felderhoff, M.; Bogdanovič, B. High temperature metal hydrides as heat storage materials for solar and related applications. Int. J. Mol. Sci. 2009, 10, 325–344. [Google Scholar] [CrossRef] [PubMed]
- Nachev, S.; de Rango, P.; Fruchart, D.; Skryabina, N.; Marty, P. Correlation between microstructural and mechanical, behavior of nanostructured MgH2 upon hydrogen cycling. J. Alloys Compd. 2015, 645, S434–S437. [Google Scholar] [CrossRef]
- Mirabile Gattia, D.; Gizer, G.; Montone, A. Effects of the compaction pressure and of the cycling process on kinetics and microstructure of compacted MgH2-based mixtures. Int. J. Hydrogen Energy 2014, 39, 9924–9930. [Google Scholar] [CrossRef]
- Mirabile Gattia, D.; Di Girolamo, G.; Montone, A. Microstructure, and kinetics evolution in MgH2-TiO2 pellets after hydrogen cycling. J. Alloys Compd. 2014, 615, S689–S692. [Google Scholar] [CrossRef]
- Mirabile Gattia, D.; Montone, A.; Pasquini, L. Microstructure and morphology changes in MgH2/expanded natural graphite pellets upon hydrogen cycling. Int. J. Hydrogen Energy 2013, 38, 1918–1924. [Google Scholar] [CrossRef]
- Mirabile Gattia, D.; Montone, A.; Di Sarcina, I.; Nacucchi, M.; De Pascalis, F.; Re, M.; Pesce, E.; Antisari, M.V. On the degradation mechanisms of Mg hydride pellets for hydrogen storage in tanks. Int. J. Hydrogen Energy 2016, 41, 9834–9840. [Google Scholar] [CrossRef]
- Chaise, A.; de Rango, P.; Marty, P.; Fruchart, D.; Miraglia, S.; Olives, R.; Garrier, S. Enhancement of hydrogen sorption in magnesium hydride using expanded natural graphite. Int. J. Hydrogen Energy 2009, 34, 8589–8596. [Google Scholar] [CrossRef]
- Lutterotti, L. Total pattern fitting for the combined size–strain–stress–texture determination in thin film diffraction. Nucl. Instrum. Methods Phys. Res. Sect. B 2010, 268, 334–340. [Google Scholar] [CrossRef]
- Mirabile Gattia, D.; Montone, A.; Di Sarcina, I. Improving magnesium based systems for efficient hydrogen storage tanks. Int. J. Hydrogen Energy 2016, 41, 14455–14460. [Google Scholar] [CrossRef]

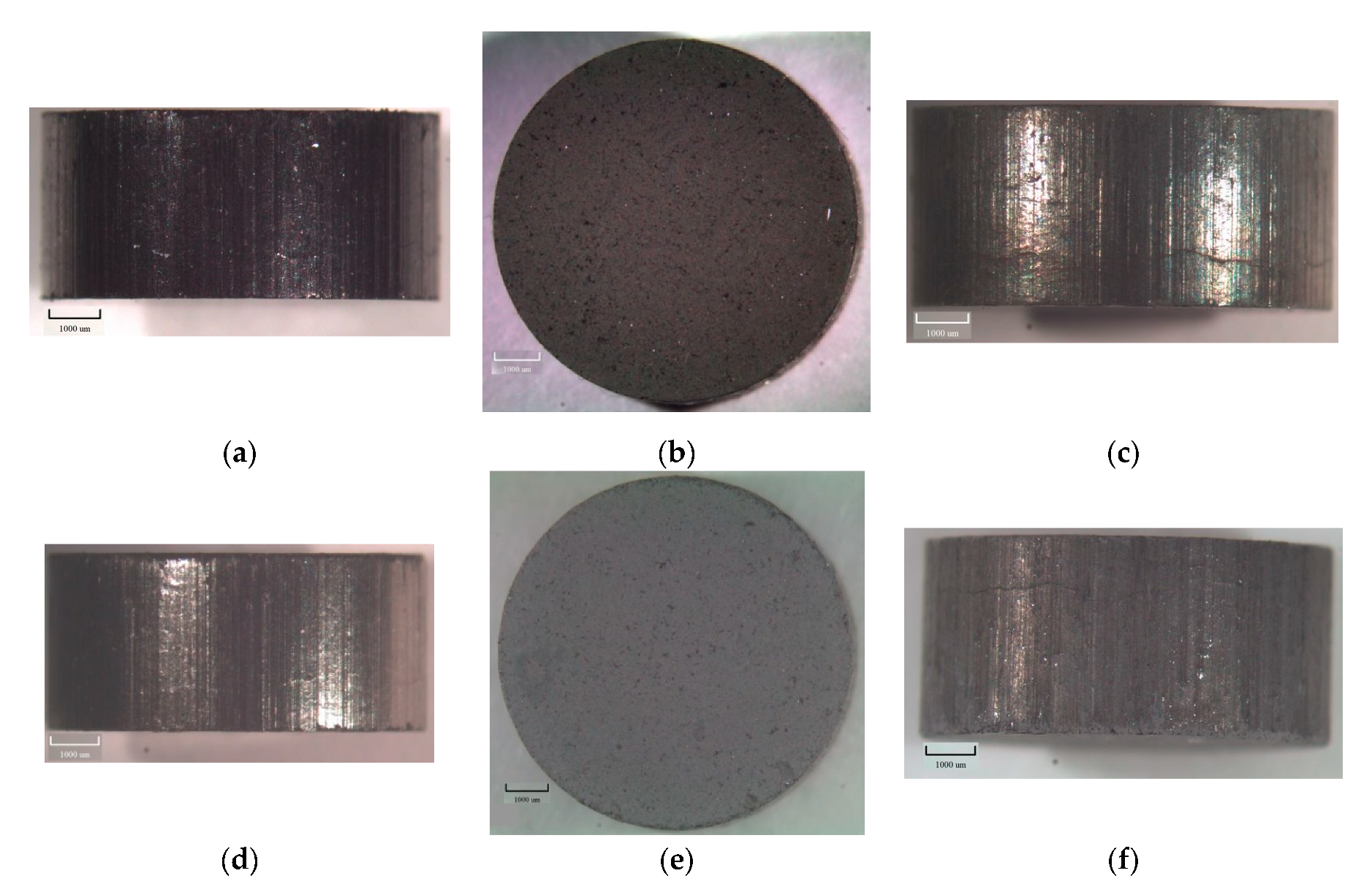



| Samples | β-MgH2 | γ-MgH2 | Fe | Mg | MgO | C | ||
|---|---|---|---|---|---|---|---|---|
| MgH2 as prepared sig = 1.866 Rwp (%) = 11.001 | Cell Parameters (Å) | a | 4.5163 (1) | – | – | 3.2093 (1) | 4.2135 (3) | – |
| b | – | – | – | – | – | – | ||
| c | 3.0208 (1) | – | – | 5.2116 (2) | – | |||
| Crystallite size (nm) | 1366 (109) | – | – | 641 (91) | 83 (3) | – | ||
| Microstrain | 0.0004 (1) | – | – | 0.0003 (1) | 0.0165 (2) | – | ||
| MgH2 milled 10 h sig = 1.885 Rwp (%) = 11.110 | Cell Parameters (Å) | a | 4.5226 (7) | 4.5244 (4) | 2.8719 (6) | 3.2893 (4) | 4.2159 (1) | – |
| b | – | 5.4269 (5) | – | – | – | – | ||
| c | 3.0262 (8) | 4.9838 (5) | – | 5.2251 (2) | – | – | ||
| Crystallite size (nm) | 15 (1) | 6 (1) | 98 (5) | 27 (6) | 99 (5) | – | ||
| Microstrain | 0.0004 (1) | 0.0003 (1) | 0.0018 (2) | 0.0095 (7) | 0.0173 (1) | – | ||
| Cycled 20 sig = 1.888 Rwp (%) = 12.745 | Cell Parameters (Å) | a | 4.5166 (1) | – | 2.8684 (2) | 3.2115 (4) | 4.2179 (7) | 2.4812 (8) |
| b | – | – | – | – | – | |||
| c | 3.0209 (9) | – | – | 5.2148 (1) | – | 6.7156 (8) | ||
| Crystallite size (nm) | 343 (9) | – | 81 (11) | 113 (39) | 53 (1) | 72 (4) | ||
| Microstrain | 0.0004 (1) | – | 0.0012 (2) | 0.0008 (3) | 0.0026 (6) | 0.0007 (6) | ||
| Cycled 45 sig = 1.941 Rwp (%) = 12.682 | Cell Parameters (Å) | a | 4.5169 (1) | – | 2.8689 (2) | 3.2114 (6) | 4.2193 (6) | 2.4831 (1) |
| b | – | – | – | – | – | – | ||
| c | 3.0210 (9) | – | – | 5.2133 (1) | – | 6.7159 (9) | ||
| Crystallite size (nm) | 412 (19) | – | 52 (6) | 220 (5) | 13 (1) | 76 (6) | ||
| Microstrain | 0.0004 (1) | – | 0.0010 (2) | 0.0003 (1) | 0.0026 (5) | 0.0010 (5) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirabile Gattia, D.; Jangir, M.; Jain, I.P. Behavior of Compacted Magnesium-Based Powders for Energy-Storage Applications. Inorganics 2020, 8, 54. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics8100054
Mirabile Gattia D, Jangir M, Jain IP. Behavior of Compacted Magnesium-Based Powders for Energy-Storage Applications. Inorganics. 2020; 8(10):54. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics8100054
Chicago/Turabian StyleMirabile Gattia, Daniele, Mukesh Jangir, and Indra Prabh Jain. 2020. "Behavior of Compacted Magnesium-Based Powders for Energy-Storage Applications" Inorganics 8, no. 10: 54. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics8100054





