Magnetic Composite Submicron Carriers with Structure-Dependent MRI Contrast
Abstract
:1. Introduction
2. Results
2.1. Magnetite Nanoparticle Synthesis
2.2. Magnetic Polymeric Submicron Core–Shells
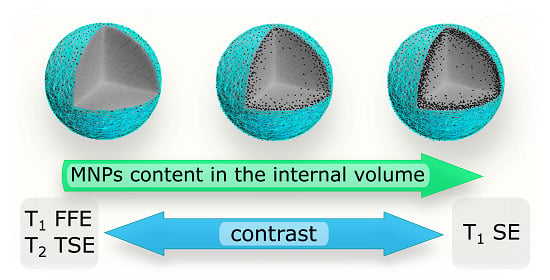
2.3. MRI Study
2.3.1. Magnetite Nanoparticle Colloid
2.3.2. Magnetic Submicron Core–Shells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Magnetite Nanoparticle Synthesis
4.3. Fabrication of Magnetic Polymeric Submicron Core–Shells
4.4. Colorimetric Titration Measurements
4.5. TEM, DLS, SEM, and EDX Characterization
4.6. Dynamics of Submicron Core–Shell Sedimentation
4.7. MRI Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roberts, D.R.; Holden, K.R. Progressive increase of T1 signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images in the pediatric brain exposed to multiple doses of gadolinium contrast. Brain Dev. 2016, 38, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Pullicino, R.; Radon, M.; Biswas, S.; Bhojak, M.; Das, K. A Review of the Current Evidence on Gadolinium Deposition in the Brain. Clin. Neuroradiol. 2018, 28, 159–169. [Google Scholar] [CrossRef] [PubMed]
- White, G.W.; Gibby, W.A.; Tweedle, M.F. Comparison of Gd(DTPA-BMA) (Omniscan) Versus Gd(HP-DO3A) (ProHance) Relative to Gadolinium Retention in Human Bone Tissue by Inductively Coupled Plasma Mass Spectroscopy. Invest. Radiol. 2006, 41, 272–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Layne, K.A.; Dargan, P.I.; Archer, J.R.H.; Wood, D.M. Gadolinium deposition and the potential for toxicological sequelae – A literature review of issues surrounding gadolinium-based contrast agents. Br. J. Clin. Pharmacol. 2018, 84, 2522–2534. [Google Scholar] [CrossRef]
- Vergauwen, E.; Vanbinst, A.-M.; Brussaard, C.; Janssens, P.; De Clerck, D.; Van Lint, M.; Houtman, A.C.; Michel, O.; Keymolen, K.; Lefevere, B.; et al. Central nervous system gadolinium accumulation in patients undergoing periodical contrast MRI screening for hereditary tumor syndromes. Hered. Cancer Clin. Pract. 2018, 16, 2. [Google Scholar] [CrossRef] [Green Version]
- Oksendal, A.N.; Hals, P.-A. Biodistribution and toxicity of MR imaging contrast media. J. Magn. Reson. Imaging 1993, 3, 157–165. [Google Scholar] [CrossRef]
- Bao, Y.; Sherwood, J.A.; Sun, Z. Magnetic iron oxide nanoparticles as T 1 contrast agents for magnetic resonance imaging. J. Mater. Chem. C 2018, 6, 1280–1290. [Google Scholar] [CrossRef]
- Di Gregorio, E.; Furlan, C.; Atlante, S.; Stefania, R.; Gianolio, E.; Aime, S. Gadolinium Retention in Erythrocytes and Leukocytes From Human and Murine Blood Upon Treatment With Gadolinium-Based Contrast Agents for Magnetic Resonance Imaging. Invest. Radiol. 2020, 55, 30–37. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, X.; Huang, J.; Wang, L.; Shen, H.; Luo, Y.; Li, Z.; Zhang, H.; Deng, Z.; Zhang, Z. Ultrasmall graphene oxide based T1 MRI contrast agent for in vitro and in vivo labeling of human mesenchymal stem cells. Nanomedicine Nanotechnology, Biol. Med. 2018, 14, 2475–2483. [Google Scholar] [CrossRef] [PubMed]
- Stark, D.D.; Weissleder, R.; Elizondo, G.; Hahn, P.F.; Saini, S.; Todd, L.E.; Wittenberg, J.; Ferrucci, J.T. Superparamagnetic iron oxide: Clinical application as a contrast agent for MR imaging of the liver. Radiology 1988, 168, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Lee, N.; Kim, H.; An, K.; Park, Y.I.; Choi, Y.; Shin, K.; Lee, Y.; Kwon, S.G.; Na, H.B.; et al. Large-Scale Synthesis of Uniform and Extremely Small-Sized Iron Oxide Nanoparticles for High-Resolution T 1 Magnetic Resonance Imaging Contrast Agents. J. Am. Chem. Soc. 2011, 133, 12624–12631. [Google Scholar] [CrossRef] [PubMed]
- Starsich, F.H.L.; Eberhardt, C.; Keevend, K.; Boss, A.; Hirt, A.M.; Herrmann, I.K.; Pratsinis, S.E. Reduced Magnetic Coupling in Ultrasmall Iron Oxide T 1 MRI Contrast Agents. ACS Appl. Bio Mater. 2018, 1, 783–791. [Google Scholar] [CrossRef]
- Shin, T.-H.; Choi, J.; Yun, S.; Kim, I.-S.; Song, H.-T.; Kim, Y.; Park, K.I.; Cheon, J. T 1 and T 2 Dual-Mode MRI Contrast Agent for Enhancing Accuracy by Engineered Nanomaterials. ACS Nano 2014, 8, 3393–3401. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-K.; Lui, C.N.P.; Chen, Y.-W.; Chou, S.-W.; Raine, E.; Chou, P.-T.; Yung, K.K.L.; Tsang, S.C.E. Engineering of Single Magnetic Particle Carrier for Living Brain Cell Imaging: A Tunable T 1 -/T 2 -/Dual-Modal Contrast Agent for Magnetic Resonance Imaging Application. Chem. Mater. 2017, 29, 4411–4417. [Google Scholar] [CrossRef]
- Szpak, A.; Fiejdasz, S.; Prendota, W.; Strączek, T.; Kapusta, C.; Szmyd, J.; Nowakowska, M.; Zapotoczny, S. T1–T2 Dual-modal MRI contrast agents based on superparamagnetic iron oxide nanoparticles with surface attached gadolinium complexes. J. Nanoparticle Res. 2014, 16, 2678. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.; Wu, A.; Chen, X. Iron Oxide Nanoparticle Based Contrast Agents for Magnetic Resonance Imaging. Mol. Pharm. 2017, 14, 1352–1364. [Google Scholar] [CrossRef]
- Sharma, V.K.; Alipour, A.; Soran-Erdem, Z.; Aykut, Z.G.; Demir, H.V. Highly monodisperse low-magnetization magnetite nanocubes as simultaneous T 1 – T 2 MRI contrast agents. Nanoscale 2015, 7, 10519–10526. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Yi, P.W.; Sun, Q.; Lei, H.; Li Zhao, H.; Zhu, Z.H.; Smith, S.C.; Lan, M.B.; Lu, G.Q.M. Ultrasmall Water-Soluble and Biocompatible Magnetic Iron Oxide Nanoparticles as Positive and Negative Dual Contrast Agents. Adv. Funct. Mater. 2012, 22, 2387–2393. [Google Scholar] [CrossRef]
- German, S.V.; Bratashov, D.N.; Navolokin, N.A.; Kozlova, A.A.; Lomova, M.V.; Novoselova, M.V.; Burilova, E.A.; Zyev, V.V.; Khlebtsov, B.N.; Bucharskaya, A.B.; et al. In vitro and in vivo MRI visualization of nanocomposite biodegradable microcapsules with tunable contrast. Phys. Chem. Chem. Phys. 2016, 18, 32238–32246. [Google Scholar] [CrossRef]
- Zeng, J.; Jing, L.; Hou, Y.; Jiao, M.; Qiao, R.; Jia, Q.; Liu, C.; Fang, F.; Lei, H.; Gao, M. Anchoring Group Effects of Surface Ligands on Magnetic Properties of Fe 3 O 4 Nanoparticles: Towards High Performance MRI Contrast Agents. Adv. Mater. 2014, 26, 2694–2698. [Google Scholar] [CrossRef]
- Sukhorukov, G.B.; Donath, E.; Davis, S.; Lichtenfeld, H.; Caruso, F.; Popov, V.I.; Möhwald, H. Stepwise polyelectrolyte assembly on particle surfaces: A novel approach to colloid design. Polym. Adv. Technol. 1998, 9, 759–767. [Google Scholar] [CrossRef]
- Romodina, M.N.; Khokhlova, M.D.; Lyubin, E.V.; Fedyanin, A.A. Direct measurements of magnetic interaction-induced cross-correlations of two microparticles in Brownian motion. Sci. Rep. 2015, 5, 10491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- German, S.V.; Novoselova, M.V.; Bratashov, D.N.; Demina, P.A.; Atkin, V.S.; Voronin, D.V.; Khlebtsov, B.N.; Parakhonskiy, B.V.; Sukhorukov, G.B.; Gorin, D.A. High-efficiency freezing-induced loading of inorganic nanoparticles and proteins into micron- and submicron-sized porous particles. Sci. Rep. 2018, 8, 17763. [Google Scholar] [CrossRef] [Green Version]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- German, S.V.; Inozemtseva, O.A.; Markin, A.V.; Metvalli, K.; Khomutov, G.B.; Gorin, D.A. Synthesis of magnetite hydrosols in inert atmosphere. Colloid J. 2013, 75, 483–486. [Google Scholar] [CrossRef]
- Westbrook, C.; Kaut, C. MRI in Practice, 4th ed.; Wiley-Blackwell: Oxford, UK, 1998; Volume 37, ISBN1 0632042052. ISBN2 9780632042050. [Google Scholar]









| Type of Sample | Core–Shell’s Structure | Amount of Fe3O4 Loaded, mg | The Mass Amount of Magnetite per Carrier, pg |
|---|---|---|---|
| S | PAH/PSS/PSH/MNPs/PAH/PSS | 0.445 ± 0.003 | 1.78 ± 0.01 |
| LCS | MNPslow@PAH/PSS/PSH/MNPs/PAH/PSS | 0.605 ± 0.003 | 2.42 ± 0.01 |
| HCS | MNPshigh@PAH/PSS/PSH/MNPs/PAH/PSS | 1.987 ± 0.033 | 7.95 ± 0.13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozlova, A.A.; German, S.V.; Atkin, V.S.; Zyev, V.V.; Astle, M.A.; Bratashov, D.N.; Svenskaya, Y.I.; Gorin, D.A. Magnetic Composite Submicron Carriers with Structure-Dependent MRI Contrast. Inorganics 2020, 8, 11. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics8020011
Kozlova AA, German SV, Atkin VS, Zyev VV, Astle MA, Bratashov DN, Svenskaya YI, Gorin DA. Magnetic Composite Submicron Carriers with Structure-Dependent MRI Contrast. Inorganics. 2020; 8(2):11. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics8020011
Chicago/Turabian StyleKozlova, Anastasiia A., Sergey V. German, Vsevolod S. Atkin, Victor V. Zyev, Maxwell A. Astle, Daniil N. Bratashov, Yulia I. Svenskaya, and Dmitry A. Gorin. 2020. "Magnetic Composite Submicron Carriers with Structure-Dependent MRI Contrast" Inorganics 8, no. 2: 11. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics8020011