1. Introduction
Two-dimensional (2D) organic–inorganic halide perovskites have attracted significant research interests owing to their wide tunability and excellent optical/electronic properties [
1,
2]. The efficiency of 2D perovskite solar cells has been increasingly improved and perovskite solar cells show tremendous advantages over silicon solar cells [
3,
4,
5]. The perovskite bulk structure has the general formula of ABX
3, where the A-site cations are normally inorganic alkali ions Cs
+, Rb
+, or can be substituted by short organic cations CH
3NH
3+ (methylammonium, MA) or CH(NH
2)
2+ (formamidinium, FA). The BX
3 are octahedrons composed of Pb
2+ (or Sn
2+) and halide ions. In contrast to the bulk perovskites, 2D halide perovskites are usually formed in organic–inorganic hybrid phases, with separated octahedral [BX
6]
4− layers jointed by long organic chains, such as
n-butylammonium (
n-BA
+), bithiophene (2T), and tetrathiophene (4T). It is expected that all-inorganic 2D layered structure is difficult to obtain as it is not so easy to separate the layers without chains in between. A case of all-inorganic 2D CsPbBr
3 has just been reported recently [
6,
7]. It is well recognized that the performance of the 2D halide perovskites solar cells is closely related to the structure, especially their microstructure. Transmission electron microscopy (TEM) has been widely used to study the crystal structure and real atomic resolution is achieved in the recent decades owing to aberration correction [
8]. However, halide perovskites are found to be electron-beam sensitive that hinders high resolution imaging [
2]. With carefully controlling of the electron dose, atomic resolution has been demonstrated on the inorganic 2D halide perovskites [
6]. However, hybrid 2D perovskites are still difficlut to be characterized as the organic portions are even more beam-sensitive. Apart from electron beam irradiation during characterization, humidity is another important factor to be considered, which results in detrimental effect on the photovoltaic efficiency and stability, hampering the wide application in solar cells [
9]. Up to now, the mechanism of the structure sensitivity to electron irradiation and water molecules has not been clearly illustrated yet. In addition, the resultant damaged products are still controversial [
6,
10,
11,
12].
Taking 2D BA2FAPb2I7 and BA2MAPb2I7 as examples, this report investigates the structural changes arising from high-energy electron irradiation, moist air, as well as temperature change, respectively. The conclusion is made based on the analysis of selected-area electron diffraction (SAED) and high-resolution transmission electron microscopy (HRTEM) under low dose-rate electron beam irradiation. The structural destruction and resultant products under electron irradiation are analyzed in detail and the effect of moist air has also been studied as well. The process of structural destruction is highly related with the bonding strength of different structural units. Furthermore, structural evolution at low temperature is also evaluated.
2. Results
As an example, the layered crystal structure of BA
2MAPb
2I
7 is shown in
Figure 1A. Different from the bulk ABX
3 formula, layered 2D structure has the general formula of A
n+1B
nX
3n+1, consisting of alternated ABX
3 and AX layers, which is also known as Ruddlesden-Popper (RP) phase [
7]. Here, the index
n = 2 and the A-site is occupied by BA
+ or MA
+ organic groups. The B-site lead (Pb) atoms are embedded in octahedral voids surrounded by iodide (I) atoms. The framework structure of lead-iodide (Pb–I) layer is formed by Pb–I octahedrons connecting with corner-shared I atoms. The Pb-I layer is separated by weakly bonded organic long chain, which is the main reason that the 2D perovskites can be mechanically exfoliated. The crystal structure shown here was solved by single crystal X-ray diffraction at 310 K (detailed in
Table S1). By substituting MA
+ with FA
+, the BA
2FAPb
2I
7 may share the same structure as BA
2MAPb
2I
7 at room temperature, and SAED index for BA
2FAPb
2I
7 and BA
2MAPb
2I
7 is based on the above crystal structure. The morphology of a 2D BA
2FAPb
2I
7 sheet with ~5 by 5 µm in length is shown in
Figure 1B. The corresponding SAED pattern in
Figure 1C depicts the perfect crystallinity. It should be noted that several diffraction spots that do not belong to the zero order Laue zone appear in the pattern owing to the large excitation error of such thin sheet. The HRTEM image along [100] the zone axis was acquired at the dose-rate of less than 50 e
− Å
−2 s
−1. In order to enhance the signal for noisy low dose data, average background subtraction filtering (ABSF) was applied to the raw image and the ABSF-filtered image is shown in
Figure 1D [
13]. Hence, clear orthogonal lattice fringes can be observed.
In the following, the effect of electron irradiation was evaluated by monitoring the structural change in sequential HRTEM images.
Figure 2A–C shows three sequential ABSF-filtered HRTEM images acquired at the dose-rate of ~180 e
− Å
−2 s
−1. The exposure time of each image is 200 ms and the raw images are shown in
Figure S1. The Fourier transform (FT) of HRTEM image reveals the frequency information in reciprocal space, where a spot in reciprocal space corresponds to a set of lattice planes in real space. The evolution of Fourier spots is easier to be distinguished compared to images and therefore it could be used as a criterion to evaluate the structural damage.
Compared to the diffraction pattern of almost intact sample (
Figure 1C), the initial disappeared spots correspond to (0
1) and (0
) plane (
Figure 2E). At this moment, the lattice in real-space image is relatively uniform (
Figure 2A). As the total dose accumulating further, the FT spots corresponding to (
), (1
1), and (0
0) planes fade away gradually (
Figure 2F). At the same time, the edge of originally uniform lattices begins to fade away in
Figure 2B. After further electron irradiation, the nearest (1
) and (1
1) spots in
Figure 2G continue to disappear, and the corresponding HRTEM image demonstrates that the uniform nanosheet is destructed to a smaller region, where the edge of the crystalline region is indicated by dotted line in
Figure 2C. By comparing with the crystal structure of R
m PbI
2 [
14], the FT pattern is consistent with the simulated SAED pattern along the zone axis [48
] and HRTEM image is also consistent with structure of PbI
2 in [48
] projection (
Figure 2D,H). Comparing the FT pattern of
Figure 2F with
Figure 2G, the nearest four {1
1} spots (6 Å) disappear and only the outer spots (3 Å) remain. The 6 Å and 3 Å lattices represent the distances of Pb–I–Pb in octahedron and Pb–I respectively. Hence, it can be inferred that finally the skeleton of Pb–I octahedron gradually collapses into small domains composed of PbI
2 when exposed to high-energy electron beam.
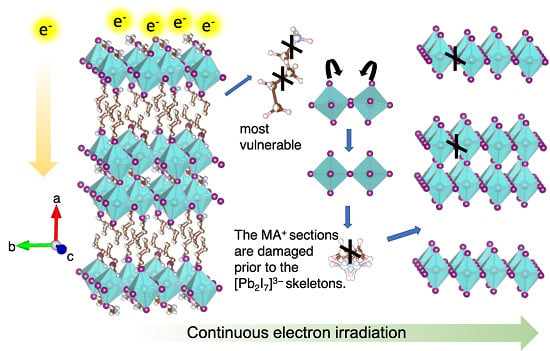
To further understand the electron irradiation-induced structural damage, structure-diffraction relationship is analyzed in detail. For such purpose, the original structure is decomposed into three sections: the long organic chains (BA
+), the short organic cations (MA
+/FA
+), and the inorganic Pb–I layer [Pb
2I
7]
3− (
Figure 3A–D). The electron diffraction from the whole structure can be considered as the summation of diffraction from each section along [100] the zone axis, whose projections are shown in
Figure 3F–I. The initial disappeared spots correspond to (0
1) planes, indicating the atoms in the (0
1) planes vanished or moved away under the electron beam. As shown in
Figure 3L,N, both the BA
+ section and the [Pb
2I
7]
3− section contribute to the (0
1) spot. For the former, given that the nitrogen atom and three out of four carbon atoms in the BA
+ section are on the (0
1) plane (
Figure 4A–F), the movements of carbon/nitrogen atoms make the long organic chains disorder and even break them into pieces. Regarding the latter, the original corner-shared octahedrons are tilted, which is exactly the reason for that the I atom columns lie in the (0
1) plane but not the (0
0) plane (
Figure 3I). If the (0
1) spots first disappear, it is simply because electron beam breaks the bonds between Pb and I, the whole skeletons of Pb–I octahedron might be collapsed and thus the low frequency information, i.e., the nearest (1
) spot (6 Å) should also disappear. Apparently, it is not the case experimentally. As such, it could be more plausible that the originally tilted octahedrons convert to non-tilted octahedrons, the I atom scattering contributes less to (0
1) spots and more to the (002)/(020) spots (
Figure 3D,E,I,J,N,O). Therefore, as for the structural collapse, the long organic chains (BA
+) are more vulnerable and could be damaged prior to the collapse of [Pb
2I
7]
3− skeletons. On this basis, even though the long organic chains are first damaged, the Pb–I octahedrons have not yet collapsed so that the HRTEM image still stays uniform (
Figure 2A). With continuous electron irradiation, the fading FT spots corresponding to (
), (
), and (
) planes are denoted by three black circles in
Figure 3M,O, which suggests that all the MA
+/FA
+, and [Pb
2I
7]
3− sections are gradually damaged. To be noted that although those three spots also exist in
Figure 3L, it is believed that the BA
+ section has already been damaged in the first step, and therefore it is not considered anymore. The last disappeared (
1) and (111) spots correspond to the MA
+/FA
+ section and also the [Pb
2I
7]
3− section (
Figure 4I). Specifically, the (0
) and (
) spots are vanished prior to the (1
1) and (1
) spots. As shown in
Figure 4G,H, the (
) plane corresponds to the lattice plane of I atom columns, implying that partial Pb–I bonds are destroyed by the electron beam. After all the organic groups damaged and partial Pb–I bonds broken, the [Pb
2I
7]
3− skeletons collapse at the end, resulting in the decomposed product of other phase such as PbI
2 (
Figure 2C).
Next, the structural damage in moist air was investigated. The low magnification image of an air-degraded sample is shown in
Figure 5A, and porous feature could be observed compared to well-preserved sample (
Figure 1B). The SAED pattern and FT of the HRTEM image show that the interplanar spacing of the damaged sample matches well with that of PbI
2 (
Figure 5B,D and
Figure 2G), suggesting that degraded structure in moist air is the same as the damaged structure under the electron beam. The difference is that the degraded crystalline domain is smaller in moist air (approximately 5~10 nm) (
Figure 5C).
Finally, the temperature effect was evaluated. It is almost becoming a common sense that lowering the temperature could protect irradiation sensitive samples from being damage, especially for materials containing organic components [
15]. In principle, low temperature does slow down the atom movements and hence prolong the stable time for the intrinsic structure. Nevertheless, we would like to remind that the liquid nitrogen condition is not suitable for all sensitive materials, and one should be careful if there is phase transformation at lower temperature. Here the BA
2FAPb
2I
7 sheet (
Figure 6A) is an example. When lowering the temperature to liquid nitrogen temperature, satellite diffraction spots appear in the diffraction pattern of BA
2FAPb
2I
7 around the main diffraction spots (
Figure 6B and
Figure 7A), being consistent with the simulated SAED of low temperature BA
2FAPb
2I
7 structure (solved at 150 K) (
Figure 7A,C) [
16]. This structure is different from the room temperature structure (
Figure 1C). Even keeping at liquid nitrogen temperature, structural damage could occur if electron irradiation accumulates to certain amount. The SAED pattern after 2 min irradiation (the accumulative dose reaches to about 5 e
− Å
−2) is shown in
Figure 6C. Although the pattern looks similar with
Figure 6B, the lattice distance has already been changed slightly (
Figure 7). It is indexed to be a different structure in different zone axis, and it is quite similar with the room temperature case (
Figure 1C). The phase transformation could be understood as illustrated in
Figure 6D–F. The low temperature is monoclinic structure (
Figure 6E) while the room temperature is orthorhombic (
Figure 6D). The low temperature structure can be considered as the room temperature structure shearing along the plane parallel to Pb–I layer and contracting along the direction perpendicular to Pb–I layer (
Figure 6F). Under the electron irradiation, BA
2FAPb
2I
7 transits gradually from monoclinic to orthorhombic structure. For the case of BA
2MAPb
2I
7, similar phenomenon was also observed.