Iron Oxide Nanoparticles: An Alternative for Positive Contrast in Magnetic Resonance Imaging
Abstract
:1. Introduction
2. T1-BASED MR
2.1. Spin Density and Relaxation Times
2.2. T1-Weighted or Positive Contrast Using Gradient and Spin Echo Sequences
3. Nanoparticles for Positive Contrast MRI
3.1. Paramagnetic Gd2O3 Nanoparticles
3.2. Paramagnetic MnO Nanoparticles
3.3. Organic Nanostructured Materials
3.4. Silica Based Nanoparticles
3.5. Liposomes
4. Iron Oxide Nanoparticles for MRI
4.1. Physicochemical Properties
4.2. Synthesis
4.2.1. Co-Precipitation
4.2.2. Thermal Decomposition
4.2.3. Polyol Synthesis
4.2.4. Microwave Assisted Synthesis
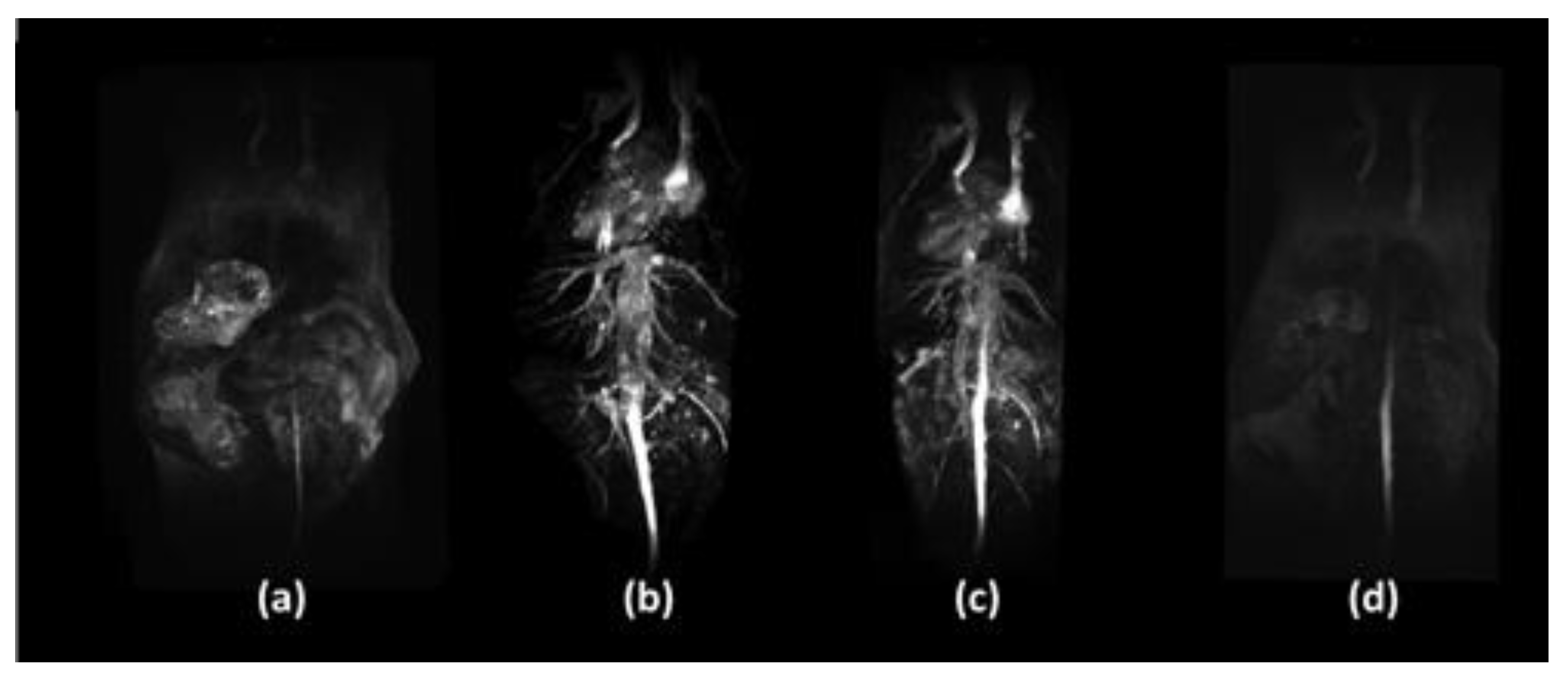
5. In Vivo Applications
5.1. Iron-Based and Iron Oxide Nanoparticles
5.2. Doped Iron Oxide Nanoparticles
5.3. Multimodal T1-Iron Oxide Nanoparticles
6. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Markl, M.; Leupold, J. Gradient echo imaging. J. Magn. Reson. Imaging 2012, 35, 1274–1289. [Google Scholar] [CrossRef] [PubMed]
- Lohrke, J.; Frenzel, T.; Endrikat, J.; Alves, F.C.; Grist, T.M.; Law, M.; Lee, J.M.; Leiner, T.; Li, K.-C.; Nikolaou, K.; et al. 25 Years of Contrast-Enhanced MRI: Developments, Current Challenges and Future Perspectives. Adv. Ther. 2016, 33, 1–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, H.B.; Hyeon, T. Nanostructured T1 MRI contrast agents. J. Mater. Chem. 2009, 19, 6267–6273. [Google Scholar] [CrossRef]
- Bridot, J.-L.; Faure, A.-C.; Laurent, S.; Riviere, C.; Billotey, C.; Hiba, B.; Janier, M.; Josserand, V.; Coll, J.-L.; Elst, L.V.; et al. Hybrid Gadolinium Oxide Nanoparticles: Multimodal Contrast Agents for in Vivo Imaging. J. Am. Chem. Soc. 2007, 129, 5076–5084. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Baek, M.J.; Choi, E.S.; Woo, S.; Kim, J.H.; Kim, T.J.; Jung, J.C.; Chae, K.S.; Chang, Y.; Lee, G.H. Paramagnetic Ultrasmall Gadolinium Oxide Nanoparticles as Advanced T1 MRI Contrast Agent: Account for Large Longitudinal Relaxivity, Optimal Particle Diameter, and In Vivo T1 MR Images. ACS Nano 2009, 3, 3663–3669. [Google Scholar] [CrossRef]
- Evanics, F.; Diamente, P.R.; van Veggel, F.C.J.M.; Stanisz, G.J.; Prosser, R.S. Water-Soluble GdF3 and GdF3/LaF3 NanoparticlesPhysical Characterization and NMR Relaxation Properties. Chem. Mater. 2006, 18, 2499–2505. [Google Scholar] [CrossRef]
- Cheung, E.N.M.; Alvares, R.D.; Oakden, W.; Chaudhary, R.; Hill, M.L.; Pichaandi, J.; Mo, G.C.H.; Yip, C.; Macdonald, P.M.; Stanisz, G.J.; et al. Polymer-Stabilized Lanthanide Fluoride Nanoparticle Aggregates as Contrast Agents for Magnetic Resonance Imaging and Computed Tomography. Chem. Mater. 2010, 22, 4728–4739. [Google Scholar] [CrossRef]
- Hifumi, H.; Yamaoka, S.; Tanimoto, A.; Citterio, D.; Suzuki, K. Gadolinium-Based Hybrid Nanoparticles as a Positive MR Contrast Agent. J. Am. Chem. Soc. 2006, 128, 15090–15091. [Google Scholar] [CrossRef]
- Shen, Z.; Fan, W.; Yang, Z.; Liu, Y.; Bregadze, V.I.; Mandal, S.K.; Yung, B.C.; Lin, L.; Liu, T.; Tang, W.; et al. Exceedingly Small Gadolinium Oxide Nanoparticles with Remarkable Relaxivities for Magnetic Resonance Imaging of Tumors. Small 2019, 15, 1903422. [Google Scholar] [CrossRef]
- Mortezazadeh, T.; Gholibegloo, E.; Alam, N.R.; Dehghani, S.; Haghgoo, S.; Ghanaati, H.; Khoobi, M. Gadolinium(III) oxide nanoparticles coated with folic acid-functionalized poly(β-cyclodextrin-co-pentetic acid) as a biocompatible targeted nano-contrast agent for cancer diagnostic: In vitro and in vivo studies. Magn. Reson. Mater. Phys. Biol. Med. 2019, 32, 487–500. [Google Scholar] [CrossRef]
- Kumar, S.; Meena, V.K.; Hazari, P.P.; Sharma, S.K.; Sharma, R.K. Rose Bengal attached and dextran coated gadolinium oxide nanoparticles for potential diagnostic imaging applications. Eur. J. Pharm. Sci. 2018, 117, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Wen, Y.; Dong, H.; Shen, A.; Zheng, X.; Li, Y.; Feng, F. A highly sensitive living probe derived from nanoparticle-remodeled neutrophils for precision tumor imaging diagnosis. Biomater. Sci. 2019, 7, 5211–5220. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Lu, X.; Yan, C.; Liu, X.; Hou, M.; Xia, Q.; Xu, Y.; Liu, R. Gastrin-releasing peptide receptor-targeted gadolinium oxide-based multifunctional nanoparticles for dual magnetic resonance/fluorescent molecular imaging of prostate cancer. Int. J. Nanomed. 2017, 12, 6787–6797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastrogiacomo, S.; Kownacka, A.E.; Dou, W.; Burke, B.P.; de Rosales, R.T.; Heerschap, A.; Jansen, J.A.; Archibald, S.J.; Walboomers, X.F. Bisphosphonate Functionalized Gadolinium Oxide Nanoparticles Allow Long-Term MRI/CT Multimodal Imaging of Calcium Phosphate Bone Cement. Adv. Healthc. Mater. 2018, 7, 1800202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Y.; Wu, C.; Wang, S.; Li, Q.; Zhang, M.; Li, J.; Xu, K. Comparative study on in vivo behavior of PEGylated gadolinium oxide nanoparticles and Magnevist as MRI contrast agent. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 547–555. [Google Scholar] [CrossRef]
- Jiang, Y.; Zheng, W. Cardiovascular toxicities upon manganese exposure. Cardiovasc. Toxicol. 2005, 5, 345–354. [Google Scholar] [CrossRef]
- Sidoryk-Wegrzynowicz, M.; Aschner, M. Manganese toxicity in the central nervous system: The glutamine/glutamate-γ-aminobutyric acid cycle. J. Intern. Med. 2013, 273, 466–477. [Google Scholar] [CrossRef] [Green Version]
- Wolf, G.; Baum, L. Cardiovascular toxicity and tissue proton T1 response to manganese injection in the dog and rabbit. Am. J. Roentgenol. 1983, 141, 193–197. [Google Scholar] [CrossRef]
- Chevallier, P.; Walter, A.; Garofalo, A.; Veksler, I.; Lagueux, J.; Begin-Colin, S.; Felder-Fleschc, D.; Fortin, M.-A. Tailored biological retention and efficient clearance of pegylated ultra-small MnO nanoparticles as positive MRI contrast agents for molecular imaging. J. Mater. Chem. B 2014, 2, 1779–1790. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Yue, T.; Xu, Y.; Xu, K.; Xu, H.; Liu, S.; Yu, J.; Huang, J. PEGylation of MnO nanoparticles via catechol-Mn chelation to improving T1-weighted magnetic resonance imaging application. J. Appl. Polym. Sci. 2015, 132, 2–9. [Google Scholar] [CrossRef]
- Wang, P.; Yang, J.; Zhou, B.; Hu, Y.; Xing, L.; Xu, F.; Shen, M.; Zhang, G.; Shi, X. Antifouling manganese oxide nanoparticles: Synthesis, characterization, and applications for enhanced MR imaging of tumors. ACS Appl. Mater. Interfaces 2017, 9, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Shao, C.; Qu, Y.; Li, S.; Gu, W.; Zheng, T.; Ye, L.; Yu, C. Folic Acid-Conjugated MnO Nanoparticles as a T1 Contrast Agent for Magnetic Resonance Imaging of Tiny Brain Gliomas. ACS Appl. Mater. Interfaces 2014, 6, 19850–19857. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Alam, I.S.; Lavdas, I.; Wylezinska-Arridge, M.; Aboagye, E.O.; Long, N.J. RGD-targeted MnO nanoparticles as T1 contrast agents for cancer imaging-the effect of PEG length in vivo. J. Mater. Chem. B 2014, 2, 868–876. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Hou, P.; Zhang, M.; Xu, K. One-pot preparation of hydrophilic manganese oxide nanoparticles as T1 nano-contrast agent for molecular magnetic resonance imaging of renal carcinoma in vitro and in vivo. Biosens. Bioelectron. 2018, 102, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gu, Z.; Kurniawan, N.; Chen, W.; Xu, Z.P. Manganese-Based Layered Double Hydroxide Nanoparticles as a T1-MRI Contrast Agent with Ultrasensitive pH Response and High Relaxivity. Adv. Mater. 2017, 29, 1700373. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, B.H.; Singh, G.; Hak, S.; Bandyopadhyay, S.; Augestad, I.L.; Peddis, D.; Sandvig, I.; Sandvig, A.; Glomm, W.R. L-DOPA-Coated Manganese Oxide Nanoparticles as Dual MRI Contrast Agents and Drug-Delivery Vehicles. Small 2016, 12, 301–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Zhang, S.; Kang, N.; Huang, J.; Lv, X.; Wen, K.; Ye, S.; Chen, Z.; Zhou, X.; Ren, L. Polydopamine-Coated Manganese Carbonate Nanoparticles for Amplified Magnetic Resonance Imaging-Guided Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 19296–19306. [Google Scholar] [CrossRef] [Green Version]
- Zhan, Y.; Shi, S.; Ehlerding, E.B.; Graves, S.A.; Goel, S.; Engle, J.W.; Liang, J.; Tian, J.; Cai, W. Radiolabeled, Antibody-Conjugated Manganese Oxide Nanoparticles for Tumor Vasculature Targeted Positron Emission Tomography and Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2017, 9, 38304–38312. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhan, W.; Li, H.; Xu, X.; Cao, X.; Zhu, S.; Liang, J.; Chen, X. In vivo dual-modality fluorescence and magnetic resonance imaging-guided lymph node mapping with good biocompatibility manganese oxide nanoparticles. Molecules 2017, 22, 2208. [Google Scholar] [CrossRef] [Green Version]
- McMahon, M.T.; Bulte, J.W.M. Two decades of dendrimers as versatile MRI agents: A tale with and without metals. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1496. [Google Scholar] [CrossRef]
- Dias, A.P.; da Silva Santos, S.; da Silva, J.V.; Parise Filho, R.; Ferreira, E.I.; El Seoud, O.; Giarolla, J. Dendrimers in the context of nanomedicine. Int. J. Pharm. 2020, 573, 118814. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Hu, K.; Cheng, Y. Tailoring the dendrimer core for efficient gene delivery. Acta Biomater. 2016, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Opina, A.C.; Wong, K.J.; Griffiths, G.L.; Turkbey, B.I.; Bernardo, M.; Nakajima, T.; Kobayashi, H.; Choyke, P.L.; Vasalatiy, O. Preparation and long-term biodistribution studies of a PAMAM dendrimer G5–Gd-BnDOTA conjugate for lymphatic imaging. Nanomedicine 2015, 10, 1423–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, S.D.; Kukowska-Latallo, J.F.; Patri, A.K.; Chen, C.; Ge, S.; Cao, Z.; Kotlyar, A.; East, A.T.; Baker, J.R. Targeted gadolinium-loaded dendrimer nanoparticles for tumor-specific magnetic renosance contrast enhancement. Int. J. Nanomedicine 2008, 3, 201–210. [Google Scholar]
- Li, F.; Yan, H.; Wang, J.; Li, C.; Wu, J.; Wu, S.; Rao, S.; Gao, X.; Jin, Q. Non-invasively differentiating extent of liver fibrosis by visualizing hepatic integrin αvβ3 expression with an MRI modality in mice. Biomaterials 2016, 102, 162–174. [Google Scholar] [CrossRef]
- Alamdari, N.H.; Alaei-Beirami, M.; Shandiz, S.A.S.; Hejazinia, H.; Rasouli, R.; Saffari, M.; Ebrahimi, S.E.S.; Assadi, A.; Ardestani, M.S. Gd3+-Asparagine-Anionic Linear Globular Dendrimer Second-Generation G2 Complexes: Novel Nanobiohybrid Theranostics. Contrast Media Mol. Imaging 2017, 2017, 3625729. [Google Scholar]
- Wang, R.; Luo, Y.; Yang, S.; Lin, J.; Gao, D.; Zhao, Y.; Liu, J.; Shi, X.; Wang, X. Hyaluronic acid-modified manganese-chelated dendrimer-entrapped gold nanoparticles for the targeted CT/MR dual-mode imaging of hepatocellular carcinoma. Sci. Rep. 2016, 6, 33844. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Bryant, H.; Shapsa, A.; Street, H.; Mani, V.; Fayad, Z.A.; Frank, J.A.; Tsimikas, S.; Briley-Saebo, K.C. Manganese G8 dendrimers targeted to oxidation-specific epitopes: In vivo MR imaging of atherosclerosis. J. Magn. Reson. Imaging 2015, 41, 797–805. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Zhang, J.; Shi, M.; Li, D.; Lu, C.; Cao, X.; Peng, C.; Mignani, S.; Majoral, J.-P.; Shi, X. Poly(amidoamine) Dendrimer-Coordinated Copper(II) Complexes as a Theranostic Nanoplatform for the Radiotherapy-Enhanced Magnetic Resonance Imaging and Chemotherapy of Tumors and Tumor Metastasis. Nano Lett. 2019, 19, 1216–1226. [Google Scholar] [CrossRef]
- Kim, S.M.; Im, G.H.; Lee, D.G.; Lee, J.H.; Lee, W.J.; Lee, I.S. Mn2+-doped silica nanoparticles for hepatocyte-targeted detection of liver cancer in T1-weighted MRI. Biomaterials 2013, 34, 8941–8948. [Google Scholar] [CrossRef]
- Li, X.; Zhao, W.; Liu, X.; Chen, K.; Zhu, S.; Shi, P.; Chen, Y.; Shi, J. Mesoporous manganese silicate coated silica nanoparticles as multi-stimuli-responsive T1-MRI contrast agents and drug delivery carriers. Acta Biomater. 2016, 30, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Pellico, J.; Ellis, C.M.; Miller, J.; Davis, J.J. Water gated contrast switching with polymer–silica hybrid nanoparticles. Chem. Commun. 2019, 55, 8540–8543. [Google Scholar] [CrossRef] [PubMed]
- Wartenberg, N.; Fries, P.; Raccurt, O.; Guillermo, A.; Imbert, D.; Mazzanti, M. A Gadolinium Complex Confined in Silica Nanoparticles as a Highly Efficient T1/T2 MRI Contrast Agent. Chem. Eur. J. 2013, 19, 6980–6983. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.L.; Kim, J.S.; Rieter, W.J.; An, H.; Lin, W.; Lin, W. Mesoporous Silica Nanospheres as Highly Efficient MRI Contrast Agents. J. Am. Chem. Soc. 2008, 130, 2154–2155. [Google Scholar] [CrossRef]
- Carniato, F.; Tei, L.; Botta, M. Gd-Based Mesoporous Silica Nanoparticles as MRI Probes. Eur. J. Inorg. Chem. 2018, 2018, 4936–4954. [Google Scholar] [CrossRef]
- Davis, J.J.; Huang, W.-Y.; Davies, G.-L. Location-tuned relaxivity in Gd-doped mesoporous silica nanoparticles. J. Mater. Chem. 2012, 22, 22848–22850. [Google Scholar] [CrossRef] [Green Version]
- Fossheim, S.L.; Fahlvik, A.K.; Klaveness, J.; Muller, R.N. Paramagnetic liposomes as MRI contrast agents: Influence of liposomal physicochemical properties on the in vitro relaxivity. Magn. Reson. Imaging 1999, 17, 83–89. [Google Scholar] [CrossRef]
- Leclercq, F.; Cohen-Ohana, M.; Mignet, N.; Sbarbati, A.; Herscovici, J.; Scherman, D.; Byk, G. Design, Synthesis, and Evaluation of Gadolinium Cationic Lipids As Tools for Biodistribution Studies of Gene Delivery Complexes. Bioconjug. Chem. 2003, 14, 112–119. [Google Scholar] [CrossRef]
- Qiu, L.H.; Zhang, J.W.; Li, S.P.; Xie, C.; Yao, Z.W.; Feng, X.Y. Molecular imaging of angiogenesis to delineate the tumor margins in glioma rat model with endoglin-targeted paramagnetic liposomes using 3T MRI. J. Magn. Reson. Imaging 2015, 41, 1056–1064. [Google Scholar] [CrossRef]
- Song, Y.; Li, W.; Meng, S.; Zhou, W.; Su, B.; Tang, L.; Zhao, Y.; Wu, X.; Yin, D.; Fan, M.; et al. Dual integrin αvβ 3 and NRP-1-Targeting Paramagnetic Liposome for Tumor Early Detection in Magnetic Resonance Imaging. Nanoscale Res. Lett. 2018, 13, 380. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.; Chen, S.; Li, H.; Zhang, Z.; Zhong, J.; Liu, M.; Zhou, X. MRI-guided liposomes for targeted tandem chemotherapy and therapeutic response prediction. Acta Biomater. 2016, 35, 260–268. [Google Scholar] [CrossRef]
- Woodside, D.G.; Tanifum, E.A.; Ghaghada, K.B.; Biediger, R.J.; Caivano, A.R.; Starosolski, Z.A.; Khounlo, S.; Bhayana, S.; Abbasi, S.; Craft, J.W., Jr.; et al. Magnetic Resonance Imaging of Atherosclerotic Plaque at Clinically Relevant Field Strengths (1T) by Targeting the Integrin α4β1. Sci. Rep. 2018, 8, 3733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieter, W.J.; Kim, J.S.; Taylor, K.M.; An, H.; Lin, W.; Tarrant, T.; Lin, W. Hybrid Silica Nanoparticles for Multimodal Imaging. Angew. Chem. Int. Ed. 2007, 46, 3680–3682. [Google Scholar] [CrossRef] [PubMed]
- Lechevallier, S.; Mauricot, R.; Gros-Dagnac, H.; Chevreux, S.; Lemercier, G.; Phonesouk, E.; Golzio, M.; Verelst, M. Silica-Based Nanoparticles as Bifunctional and Bimodal Imaging Contrast Agents. Chempluschem 2017, 82, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Mignot, A.; Truillet, C.; Lux, F.; Sancey, L.; Louis, C.; Denat, F.; Boschetti, F.; Bocher, L.; Gloter, A.; Stéphan, O.; et al. A Top-Down Synthesis Route to Ultrasmall Multifunctional Gd-Based Silica Nanoparticles for Theranostic Applications. Chem. Eur. J. 2013, 19, 6122–6136. [Google Scholar] [CrossRef]
- Gizzatov, A.; Hernández-Rivera, M.; Keshishian, V.; Mackeyev, Y.; Law, J.J.; Guven, A.; Sethi, R.; Qu, F.F.; Muthupillai, R.; da Graça Cabreira-Hansen, M.; et al. Surfactant-free Gd3+-ion-containing carbon nanotube MRI contrast agents for stem cell labeling. Nanoscale 2015, 7, 12085–12091. [Google Scholar] [CrossRef] [Green Version]
- Servant, A.; Jacobs, I.; Bussy, C.; Fabbro, C.; Da Ros, T.; Pach, E.; Ballesteros, B.; Prato, M.; Nicolay, K.; Kostarelos, K. Gadolinium-functionalised multi-walled carbon nanotubes as a T1 contrast agent for MRI cell labelling and tracking. Carbon 2016, 97, 126–133. [Google Scholar] [CrossRef]
- Moghaddam, S.E.; Hernández-Rivera, M.; Zaibaq, N.G.; Ajala, A.; da Graça Cabreira-Hansen, M.; Mowlazadeh-Haghighi, S.; Willerson, J.T.; Perin, E.C.; Muthupillai, R.; Wilson, L.J. A New High-Performance Gadonanotube-Polymer Hybrid Material for Stem Cell Labeling and Tracking by MRI. Contrast Media Mol. Imaging 2018, 2018, 2853736. [Google Scholar] [CrossRef]
- Richard, C.; Doan, B.T.; Beloeil, J.C.; Bessodes, M.; Tóth, É.; Scherman, D. Noncovalent Functionalization of Carbon Nanotubes with Amphiphilic Gd3+ Chelates: Toward Powerful T1 and T2 MRI Contrast Agents. Nano Lett. 2008, 8, 232–236. [Google Scholar] [CrossRef]
- Rieter, W.J.; Taylor, K.M.L.; An, H.; Lin, W.; Lin, W. Nanoscale Metal−Organic Frameworks as Potential Multimodal Contrast Enhancing Agents. J. Am. Chem. Soc. 2006, 128, 9024–9025. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Zhou, J.; Chen, R.; Shi, R.; Zhao, G.; Xia, G.; Li, R.; Liu, Z.; Tian, J.; Wang, H.; et al. Controllable synthesis of dual-MOFs nanostructures for pH-responsive artemisinin delivery, magnetic resonance and optical dual-model imaging-guided chemo/photothermal combinational cancer therapy. Biomaterials 2016, 100, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.L.; Rieter, W.J.; Lin, W. Manganese-Based Nanoscale Metal−Organic Frameworks for Magnetic Resonance Imaging. J. Am. Chem. Soc. 2008, 130, 14358–14359. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Chen, Y.; Tao, C.; Tian, Q.; An, L.; Lin, J.; Tian, Q.; Yang, H.; Yang, S. Mn–Porphyrin-Based Metal–Organic Framework with High Longitudinal Relaxivity for Magnetic Resonance Imaging Guidance and Oxygen Self-Supplementing Photodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 41946–41956. [Google Scholar] [CrossRef] [PubMed]
- Mulder, W.J.M.; Strijkers, G.J.; van Tilborg, G.A.F.; Griffioen, A.W.; Nicolay, K. Lipid-based nanoparticles for contrast-enhanced MRI and molecular imaging. NMR Biomed. 2006, 19, 142–164. [Google Scholar] [CrossRef]
- Magin, R.L.; Wright, S.M.; Niesman, M.R.; Chan, H.C.; Swartz, H.M. Liposome delivery of NMR contrast agents for improved tissue imaging. Magn. Reson. Med. 1986, 3, 440–447. [Google Scholar] [CrossRef]
- Koenig, S.H.; Brown, R.D.; Kurland, R.; Ohkit, S. Relaxivity and binding of Mn2+ ions in solutions of phosphatidylserine vesicles. Magn. Reson. Med. 1988, 7, 133–142. [Google Scholar] [CrossRef]
- Devoisselle, J.M.; Vion-Dury, J.; Galons, J.P.; Confort-Gouny, S.; Coustaut, D.; Canioni, P.; Cozzone, P.J. Entrapment of Gadolinium-DTPA in Liposomes: Characterization of Vesicles by P-31 NMR Spectroscopy. Investig. Radiol. 1988, 23, 719–724. [Google Scholar] [CrossRef]
- Tilcock, C.; Unger, E.; Cullis, P.; MacDougall, P. Liposomal Gd-DTPA: Preparation and characterization of relaxivity. Radiology 1989, 171, 77–80. [Google Scholar] [CrossRef]
- Kabalka, G.W.; Davis, M.A.; Moss, T.H.; Buonocore, E.; Hubner, K.; Holmberg, E.; Maruyama, K.; Huang, L. Gadolinium-labeled liposomes containing various amphiphilic Gd-DTPA derivatives: Targeted MRI contrast enhancement agents for the liver. Magn. Reson. Med. 1991, 19, 406–415. [Google Scholar] [CrossRef]
- Kabalka, G.W.; Buonocore, E.; Hubner, K.; Davis, M.; Huang, L. Gadolinium-labeled liposomes containing paramagnetic amphipathic agents: Targeted MRI contrast agents for the liver. Magn. Reson. Med. 1988, 8, 89–95. [Google Scholar] [CrossRef]
- Trubetskoy, V.S.; Cannillo, J.A.; Milshtein, A.; Wolf, G.L.; Torchilin, V.P. Controlled delivery of Gd-containing liposomes to lymph nodes: Surface modification may enhance MRI contrast properties. Magn. Reson. Imaging 1995, 13, 31–37. [Google Scholar] [CrossRef]
- Bertini, I.; Bianchini, F.; Calorini, L.; Colagrande, S.; Fragai, M.; Franchi, A.; Gallo, O.; Gavazzi, C.; Luchinat, C. Persistent contrast enhancement by sterically stabilized paramagnetic liposomes in murine melanoma. Magn. Reson. Med. 2004, 52, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Sipkins, D.A.; Cheresh, D.A.; Kazemi, M.R.; Nevin, L.M.; Bednarski, M.D.; Li, K.C. Detection of tumor angiogenesis in vivo by αvβ3-targeted magnetic resonance imaging. Nat. Med. 1998, 4, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Aryal, M.; Papademetriou, I.; Zhang, Y.Z.; Power, C.; McDannold, N.; Porter, T. MRI Monitoring and Quantification of Ultrasound-Mediated Delivery of Liposomes Dually Labeled with Gadolinium and Fluorophore through the Blood-Brain Barrier. Ultrasound Med. Biol. 2019, 45, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Laurent, S.; Jo, Y.S.; Roch, A.; Mikhaylova, M.; Bhujwalla, Z.M.; Muller, R.N.; Muhammed, M.A. High-Performance Magnetic Resonance ImagingT2 Contrast Agent. Adv. Mater. 2007, 19, 1874–1878. [Google Scholar] [CrossRef]
- Bao, Y.; Sherwood, J.A.; Sun, Z. Magnetic iron oxide nanoparticles as T1 contrast agents for magnetic resonance imaging. J. Mater. Chem. C 2018, 6, 1280–1290. [Google Scholar] [CrossRef]
- Tao, C.; Zheng, Q.; An, L.; He, M.; Lin, J.; Tian, Q.; Yang, S. T1-Weight Magnetic Resonance Imaging Performances of Iron Oxide Nanoparticles Modified with a Natural Protein Macromolecule and an Artificial Macromolecule. Nanomaterials 2019, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Bruns, O.T.; Kaul, M.G.; Hansen, E.C.; Barch, M.; Wiśniowska, A.; Chen, O.; Chen, Y.; Li, N.; Okada, S.; et al. Exceedingly small iron oxide nanoparticles as positive MRI contrast agents. Proc. Natl. Acad. Sci. USA 2017, 114, 2325–2330. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Zhang, X.; Skallberg, A.; Liu, Y.; Hu, Z.; Mei, X.; Uvdal, K. One-step synthesis of water-dispersible ultra-small Fe3O4 nanoparticles as contrast agents for T1 and T2 magnetic resonance imaging. Nanoscale 2014, 6, 2953. [Google Scholar] [CrossRef]
- Illés, E.; Szekeres, M.; Tóth, I.Y.; Farkas, K.; Földesi, I.; Szabó, Á.; Iván, B.; Tombácz, E. PEGylation of Superparamagnetic Iron Oxide Nanoparticles with Self-Organizing Polyacrylate-PEG Brushes for Contrast Enhancement in MRI Diagnosis. Nanomaterials 2018, 8, 776. [Google Scholar] [CrossRef] [Green Version]
- Pellico, J.; Ruiz-Cabello, J.; Fernández-Barahona, I.; Gutiérrez, L.; Lechuga-Vieco, A.V.; Enríquez, J.A.; Morales, M.P.; Herranz, F. One-Step Fast Synthesis of Nanoparticles for MRI: Coating Chemistry as the Key Variable Determining Positive or Negative Contrast. Langmuir 2017, 33, 10239–10247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alipour, A.; Soran-Erdem, Z.; Utkur, M.; Sharma, V.K.; Algin, O.; Saritas, E.U.; Demir, H.V. A new class of cubic SPIONs as a dual-mode T1 and T2 contrast agent for MRI. Magn. Reson. Imaging 2018, 49, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Chan, N.; Laprise-Pelletier, M.; Chevallier, P.; Bianchi, A.; Fortin, M.A.; Oh, J.K. Multidentate block-copolymer-stabilized ultrasmall superparamagnetic iron oxide nanoparticles with enhanced colloidal stability for magnetic resonance imaging. Biomacromolecules 2014, 15, 2146–2156. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Jia, Q.; Li, Y.; Gao, M. Facile synthesis of ultrasmall PEGylated iron oxide nanoparticles for dual-contrast T1- and T2-weighted magnetic resonance imaging. Nanotechnology 2011, 22, 245604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, M.Z.; Ma, X.; Chen, T.; Ren, W.; Xiang, L.; Wu, A. Silica-coated super-paramagnetic iron oxide nanoparticles (SPIONPs): A new type contrast agent of T1 magnetic resonance imaging (MRI). J. Mater. Chem. B 2015, 3, 5172–5181. [Google Scholar] [CrossRef]
- Jung, H.; Park, B.; Lee, C.; Cho, J.; Suh, J.; Park, J.; Kim, Y.; Kim, J.; Cho, G.; Cho, H. Dual MRI T1 and T2(*) contrast with size-controlled iron oxide nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Lee, N.; Kim, H.; An, K.; Park, Y.I.; Choi, Y.; Shin, K.; Lee, Y.; Kwon, S.G.; Na, H.B.; et al. Large-scale synthesis of uniform and extremely small-sized iron oxide nanoparticles for high-resolution T1 magnetic resonance imaging contrast agents. J. Am. Chem. Soc. 2011, 133, 12624–12631. [Google Scholar] [CrossRef]
- Li, F.; Liang, Z.; Liu, J.; Sun, J.; Hu, X.; Zhao, M.; Liu, J.; Bai, R.; Kim, D.; Sun, X.; et al. Dynamically Reversible Iron Oxide Nanoparticle Assemblies for Targeted Amplification of T1-Weighted Magnetic Resonance Imaging of Tumors. Nano Lett. 2019, 19, 4213–4220. [Google Scholar] [CrossRef]
- Li, P.; Chevallier, P.; Ramrup, P.; Biswas, D.; Vuckovich, D.; Fortin, M.A.; Oh, J.K. Mussel-Inspired Multidentate Block Copolymer to Stabilize Ultrasmall Superparamagnetic Fe3O4 for Magnetic Resonance Imaging Contrast Enhancement and Excellent Colloidal Stability. Chem. Mater. 2015, 27, 7100–7109. [Google Scholar] [CrossRef]
- Li, Z.; Yi, P.W.; Sun, Q.; Lei, H.; Li Zhao, H.; Zhu, Z.H.; Smith, S.C.; Lan, M.B.; Lu, G.Q. Ultrasmall Water-Soluble and Biocompatible Magnetic Iron Oxide Nanoparticles as Positive and Negative Dual Contrast Agents. Adv. Funct. Mater. 2012, 22, 2387–2393. [Google Scholar] [CrossRef]
- Liu, C.L.; Peng, Y.K.; Chou, S.W.; Tseng, W.H.; Tseng, Y.J.; Chen, H.C.; Hsiao, J.K.; Chou, P.T. One-Step, Room-Temperature Synthesis of Glutathione-Capped Iron-Oxide Nanoparticles and their Application in In Vivo T1-Weighted Magnetic Resonance Imaging. Small 2014, 10, 3962–3969. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yang, J.; Yan, Y.; Li, J.; Shen, M.; Zhang, G.; Mignanic, S.; Shi, X. RGD-functionalized ultrasmall iron oxide nanoparticles for targeted T1-weighted MR imaging of gliomas. Nanoscale 2015, 7, 14538–14546. [Google Scholar] [CrossRef] [PubMed]
- Macher, T.; Totenhagen, J.; Sherwood, J.; Qin, Y.; Gurler, D.; Bolding, M.S.; Bao, Y. Ultrathin iron oxide nanowhiskers as positive contrast agents for magnetic resonance imaging. Adv. Funct. Mater. 2015, 25, 490–494. [Google Scholar] [CrossRef]
- Shen, Z.; Chen, T.; Ma, X.; Ren, W.; Zhou, Z.; Zhu, G.; Zhang, A.; Liu, Y.; Song, J.; Li, Z.; et al. Multifunctional Theranostic Nanoparticles Based on Exceedingly Small Magnetic Iron Oxide Nanoparticles for T1-Weighted Magnetic Resonance Imaging and Chemotherapy. ACS Nano 2017, 11, 10992–11004. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.H.; Bao, J.F.; Wang, D.; Wang, Y.X.; Chen, Z.W.; Ren, L.; Zhou, X.; Ke, X.B.; Chen, M.; Yang, A.Q. One-step synthesis of monodisperse, water-soluble ultra-small Fe3O4 nanoparticles for potential bio-application. Nanoscale 2013, 5, 2133–2141. [Google Scholar] [CrossRef]
- Taboada, E.; Rodríguez, E.; Roig, A.; Oró, J.; Roch, A.; Muller, R.N. Relaxometric and Magnetic Characterization of Ultrasmall Iron Oxide Nanoparticles with High Magnetization. Evaluation as Potential T1 Magnetic Resonance Imaging Contrast Agents for Molecular Imaging. Langmuir 2007, 23, 4583–4588. [Google Scholar] [CrossRef]
- Tromsdorf, U.I.; Bruns, O.T.; Salmen, S.C.; Beisiegel, U.; Weller, H. A Highly Effective, Nontoxic T1 MR Contrast Agent Based on Ultrasmall PEGylated Iron Oxide Nanoparticles. Nano Lett. 2009, 9, 4434–4440. [Google Scholar] [CrossRef]
- Vangijzegem, T.; Stanicki, D.; Boutry, S.; Paternoster, Q.; Vander Elst, L.; Muller, R.N.; Laurent, S. VSION as high field MRI T1 contrast agent: Evidence of their potential as positive contrast agent for magnetic resonance angiography. Nanotechnology 2018, 29, 265103. [Google Scholar] [CrossRef]
- Fernández-Barahona, I.; Gutiérrez, L.; Veintemillas-Verdaguer, S.; Pellico, J.; Morales MD, P.; Catala, M.; del Pozo, M.A.; Ruiz-Cabello, J.; Herranz, F. Cu-Doped Extremely Small Iron Oxide Nanoparticles with Large Longitudinal Relaxivity: One-Pot Synthesis and in Vivo Targeted Molecular Imaging. ACS Omega 2019, 4, 2719–2727. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Zhou, Z.; Liu, H.; Wu, C.; Zhang, H.; Huang, G.; Ai, H.; Gao, J. Europium-engineered iron oxide nanocubes with high T1 and T2 contrast abilities for MRI in living subjects. Nanoscale 2015, 7, 6843–6850. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Ren, W.; Zheng, J.; Cui, P.; Wu, A. Ultrasmall water-soluble metal-iron oxide nanoparticles as T1-weighted contrast agents for magnetic resonance imaging. Phys. Chem. Chem. Phys. 2012, 14, 2631–2636. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Russek, S.E.; Zabow, G.; Sun, F.; Mohapatra, J.; Keenan, K.E.; Boss, M.A.; Zeng, H.; Liu, P.J.; Viert, A.; et al. Large T1 contrast enhancement using superparamagnetic nanoparticles in ultra-low field MRI. Sci. Rep. 2018, 8, 11863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.; Hira Zafar, M.Z.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, C.; Chen, Y.; Wang, D.; Cai, Y.; Zheng, Q.; An, L.; Lin, J.; Tian, Q.; Yang, S. Macromolecules with Different Charges, Lengths, and Coordination Groups for the Coprecipitation Synthesis of Magnetic Iron Oxide Nanoparticles as T1 MRI Contrast Agents. Nanomaterials 2019, 9, 699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseyphus, R.J.; Kodama, D.; Matsumoto, T.; Sato, Y.; Jeyadevan, B.; Tohji, K. Role of polyol in the synthesis of Fe particles. J. Magn. Magn. Mater. 2007, 310, 2393–2395. [Google Scholar] [CrossRef]
- Hu, F.; MacRenaris, K.W.; Waters, E.A.; Liang, T.; Schultz-Sikma, E.A.; Eckermann, A.L.; Meade, T.J. Ultrasmall, Water-Soluble Magnetite Nanoparticles with High Relaxivity for Magnetic Resonance Imaging. J. Phys. Chem. C 2009, 113, 20855–20860. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Barahona, I.; Muñoz-Hernando, M.; Herranz, F. Microwave-Driven Synthesis of Iron-Oxide Nanoparticles for Molecular Imaging. Molecules 2019, 24, 1224. [Google Scholar] [CrossRef] [Green Version]
- Bhavesh, R.; Lechuga-Vieco, A.V.; Ruiz-Cabello, J.; Herranz, F. T1-MRI Fluorescent Iron Oxide Nanoparticles by Microwave Assisted Synthesis. Nanomaterials 2015, 5, 1880–1890. [Google Scholar] [CrossRef] [Green Version]
- Pellico, J.; Ruiz-Cabello, J.; Saiz-Alía, M.; del Rosario, G.; Caja, S.; Montoya, M.; Fernández de Manuel, L.; Morales, M.P.; Gutiérre, L.; Galiana, B.; et al. Fast synthesis and bioconjugation of 68Ga core-doped extremely small iron oxide nanoparticles for PET/MR imaging. Contrast Media Mol. Imaging 2016, 11, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Ju, K.Y.; Lee, J.W.; Im, G.H.; Lee, S.; Pyo, J.; Park, S.B.; Lee, J.H.; Lee, J.K. Bio-inspired, melanin-like nanoparticles as a highly efficient contrast agent for T1-weighted magnetic resonance imaging. Biomacromolecules 2013, 14, 3491–3497. [Google Scholar] [CrossRef]
- Peng, Y.K.; Liu, C.L.; Chen, H.C.; Chou, S.W.; Tseng, W.H.; Tseng, Y.J.; Kang, C.C.; Hsiao, J.K.; Chou, P.T. Antiferromagnetic iron nanocolloids: A new generation in vivo T1 mri contrast agent. J. Am. Chem. Soc. 2013, 135, 18621–18628. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Meng, Q.; Chen, Y.; Xu, P.; Zhang, S.; Li, Y.; Zhang, L.; Wang, M.; Yao, H.; Shi, J. Ultrasmall confined Iron oxide nanoparticle MSNs as a pH-responsive theranostic platform. Adv. Funct. Mater. 2014, 24, 4273–4283. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.X.; Sun, Q.; Zhao, H.L.; Lei, H.; Lan, M.B. Ultrasmall Manganese Ferrite Nanoparticles as Positive Contrast Agent for Magnetic Resonance Imaging. Adv. Healthc. Mater. 2013, 2, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Li, H.; Chen, J.; Zhao, Z.; Yang, L.; Chi, X.; Chen, Z.; Wang, X.; Gao, J. Tunable T1 and T2 contrast abilities of manganese-engineered iron oxide nanoparticles through size control. Nanoscale 2014, 6, 10404–10412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cao, Y.; Wang, L.; Ma, Y.; Tu, X.; Zhang, Z. Manganese doped iron oxide theranostic nanoparticles for combined T1 magnetic resonance imaging and photothermal therapy. ACS Appl. Mater. Interfaces 2015, 7, 4650–4658. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron Oxide Based Nanoparticles for Multimodal Imaging and Magnetoresponsive Therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef] [PubMed]
- Pellico, J.; Fernández-Barahona, I.; Benito, M.; Gaitán-Simón, Á.; Gutiérrez, L.; Ruiz-Cabello, J.; Herranz, F. Unambiguous detection of atherosclerosis using bioorthogonal nanomaterials. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Sandiford, L.; Phinikaridou, A.; Protti, A.; Meszaros, L.K.; Cui, X.; Yan, Y.; Frodsham, G.; Williamson, P.A.; Gaddum, N.; Botnar, R.M.; et al. Bisphosphonate-anchored pegylation and radiolabeling of superparamagnetic iron oxide: Long-circulating nanoparticles for in vivo multimodal (T1 MRI-SPECT) imaging. ACS Nano 2013, 7, 500–512. [Google Scholar] [CrossRef]
- Wang, G.; Gao, W.; Zhang, X.; Mei, X. Au Nanocage Functionalized with Ultra-small Fe3O4 Nanoparticles for Targeting T1–T2Dual MRI and CT Imaging of Tumor. Sci. Rep. 2016, 6, 28258. [Google Scholar] [CrossRef] [Green Version]
- Pellico, J.; Ellis, C.M.; Davis, J.J. Nanoparticle-Based Paramagnetic Contrast Agents for Magnetic Resonance Imaging. Contrast Media Mol. Imaging 2019, 2019, 1845637. [Google Scholar] [CrossRef]




| 1.5 T | 3 T | ||||
|---|---|---|---|---|---|
| Tissue | T1 (ms) | T2 (ms) | T1 (ms) | T2 (ms) | |
| Brain | Grey matter | 1150 | 100 | 1600 | 70 |
| White matter | 800 | 80 | 1100 | 60 | |
| CSF | 4500 | 2200 | |||
| Skeletal muscle | 1000 | 35 | 1400 | 30 | |
| Fat | 250 | 60 | |||
| Blood | 1400 | 290 | 1900 | 275 | |
| Liver | 580 | 55 | 810 | 56 | |
| Cardiac muscle | 1030 | 42 | 1400 | 47 | |
| Nanomaterial | Composition | DH Size (nm) | r1 (mM−1 s−1) | B0 (T) | Ref. |
|---|---|---|---|---|---|
| Paramagnetic inorganic NPs-Gadolinium | Core-shell Gd2O3@polisiloxane | 3.3 ± 0.8 | 8.8 | 7 | [4] |
| d-glucuronic acid-coated Gd2O3 | 1 | 9.9 | 1.5 | [5] | |
| Citrate-coated GdF3, AEP-coated GdF3/LaF3 | 129.3 | 8.8 ± 0.2 | 3 | [6] | |
| PAA25-stabilized GdF3/CeF3 NPAs | 70 | 40 ± 2 | 1.5 | [7] | |
| PGP/dextran-K01 | 23.2 ± 7.8 | 13.9 | 0.5 | [8] | |
| ES-GON-PAA | <2 | 70.2 ± 1.8 | 1.5 | [9] | |
| Gd2O3@PCD-FA | 131 ± 4.6 | 3.95 | 3 | [10] | |
| Gd2O3-FI-PEG-BBN | 52.3 | 4.23 | 3 | [13] | |
| Bisphosphonate-functionalised Gd2O3 | 70 | 15.41 | 3 | [14] | |
| PEG-Gd2O3 | 36.35 ± 1.9 | 29 | 3 | [15] | |
| Paramagnetic inorganic NPs-Manganese | MnO@PDn | 24.8 ± 0.2 | 4.4 | 1.41 | [19] |
| mPEG-SA-dopamine-MnO | 120 | 16.14 | 3 | [20] | |
| l-cysteine-functionalised PEG-coated Mn3O4 | 213.3 ± 2.4 | 3.66 | 0.5 | [21] | |
| FA-TETT-MnO | 122 | 4.83 | 7 | [22] | |
| MnO@AUA@PEG5000@RGD | 56.7 ± 13.2 | 1.44 | 9.4 | [23] | |
| PEG-MnO | 15.08 ± 2.7 | 12.94 | 3 | [24] | |
| Mn-LDH | 48 | 9.48 | - | [25] | |
| MnCO3@polydopamine | 173 | 8.3 | 7 | [27] | |
| NOTA-Mn3O4@PEG-TRC105 | 32.6 ± 4.5 | 0.54 | 4.7 | [28] | |
| Mn3O4@PEG-Cy7.5 | 10 ± 2.3 | 0.53 | 7 | [29] | |
| Dendrimers | PAMAM G5-BnDOTA-Gd | 6.5 | 12.98 | 3 | [33] |
| Folic acid-G5-DOTA-Gd | - | 26 ± 0.06 | 2 | [34] | |
| Den-cRGD-DOTA-Gd | 13.2 | 7.1 ± 0.3 | 4.7 | [35] | |
| Gd3+-G2-Gd-Aspargine | 90 | 1.5 | [36] | ||
| (Au)100G5.NH2-FI-DOTA(Mn)-HA | 245.3 | 5.42 | 0.5 | [37] | |
| PAMAM G8-DTPA-Mn | 13.3 ± 1.2 | 3.5 ± 0.1 | 1.5 | [38] | |
| G5.NHAc-Pyr/Cu(II) | 153.2 ± 4.6 | 0.7024 | 0.5 | [39] | |
| Liposomes | DPPC/DPPG Gd-Liposomes | 72 ± 6 | 1.13 | 0.5 | [47] |
| MCO-I-68-Gd/DNA liposomes | 150 | [48] | |||
| Mab-Gd-SLs | 129.9 ± 40.9 | 8.06 | 1.5 | [49] | |
| RGD- and ATWLPPR- functionalised Gd-liposomes | 89.9 | ~6 | 3 | [50] | |
| RGD-CPGd-L | 128 | 4.24 | 11.7 | [51] | |
| THI0567-targeted liposomal-Gd | 150–250 | 2 × 105/particle | 1 | [52] | |
| Silica NPs | Mn-SiO2 | 25 ± 2 | 6.7 | 3 | [40] |
| Doxorubicin-loaded SiO2@MnSiO3 | 150 | 4.34 | 3 | [41] | |
| Silyated Gd complex-coated [Ru(bpy)3]Cl2 | 37 | 19.7 | 3 | [53] | |
| Gd-Si-DTTA | 75 | 28.8 | 3 | [44] | |
| Gd-DOTA-MSNs | 66.3 ± 6.6 | 33.57 ± 1.29 | 7 | [46] | |
| Gd-DTPA-334 | 20 ± 2 | 18.7 | 0.5 | [54] | |
| SRPs | 8.3 | 11.9 | 1.5 | [55] | |
| Carbon nanotubes (CNTs) | Gd ultrashort single-walled CNTs | - | 90 | 1.5 | [56] |
| Gd-MWNT | - | 6.61 | 7 | [57] | |
| PAA-GNTs | - | 150 | 1.5 | [58] | |
| MWNT/GdL | - | 50.3 | 0.5 | [59] | |
| Metal–organic frameworks (MOFs) | Eu-, Gd-, Tb- doped MOFs | 100 × 35 | 35.8 | 3 | [60] |
| Core-shell PB@MIL-100(Fe) | 100 | 1.3 | 3 | [61] | |
| C(RGDfK)-MnMOFs | 50–100 × 750 | 4.0 | 9.4 | [62] | |
| PCN-222(Mn) | 241 | 35.3 | 1 | [63] |
| Sample | DH (nm) | Core Size (nm) | r1 (mM−1 s−1) | r2 (mM−1 s−1) | B0 (T) | (ref) |
|---|---|---|---|---|---|---|
| Cubic IONP | 18 | 11 | 3.4 | 36.8 | 3 | [82] |
| MDBC-USPIO | 24 | 3.4 | 4.8 | 22.56 | 1.5 | [83] |
| Pegylated SPIONs | 10.1 | 5.4 | 19.7 | 39.5 | 1.5 | [84] |
| Fe3O4@SiO2 | 30 - 40 | 4 | 1.2 | 7.8 | 3 | [85] |
| SPION | 20 ± 7 | 5–10 | 13.31 | 40.90 | 1.4 | [86] |
| ESIONs | - | 3 | 4.78 | 29.25 | 3 | [87] |
| IONAs | 17 | 9 | 5.1 | 21.3 | 3 | [88] |
| Cat-MDBC/USNP | 20 | 3.4 ± 1.8 | 6.8 | 37.1 | 1.4 | [89] |
| UMIONs | 7.5 | 3.3 ± 0.5 | 8.3 | 35.1 | 4.7 | [90] |
| GSH-IO NPs | 4.19 ± 0.31 | 3.72 ± 0.12 | 3.63 | 8.28 | 4.7 | [91] |
| Fe3O4-PEG-RGD | 212.5 | 2.7 ± 0.2 | 1.4 | - | 0.5 | [92] |
| UTIO-nanowhiskers | - | 2 × 20 | 6.13 | 11.15 | 1.4 | [93] |
| C-ESION120 | 7.9 | 4.2 | 11.9 | 22.9 | 1.5 | [81] |
| ES-MION3 | - | 3.6 | 8.8 | 22.7 | 1.5 | [94] |
| Ultrasmall Fe3O4 | - | 1.9 | 1.41 | 2.87 | 7 | [95] |
| Fe2O3-water | 8 ± 2 | 4.9 ± 0.6 | 17.6 | 35.8 | 1.5 | [96] |
| Fe2O3-Citrate | 18 ± 4 | 5 ± 1 | 14.5 | 66.9 | 1.5 | [96] |
| Fe3O4-PMAA-PTTM | - | 4.34 ± 1.54 | 24.2 | 67.2 | 0.5 | [77] |
| Fe3O4-PEG1100 | 10–15 | 4 | 7.3 | 17.5 | 1.4 | [97] |
| PEG750-VSION | 19.8 | 3.5 ± 0.6 | 1.74 | 40.6 | 9.4 | [98] |
| PEG2000-VSION | 22.2 | 3.5 ± 0.6 | 1.12 | 31.1 | 9.4 | [98] |
| Ultrasmall Fe3O4 | 5.8 | 1.7 | 8.20 | 16.67 | 1.4 | [79] |
| Ultrasmall Fe3O4 | 5.8 | 2.2 | 6.15 | 28.62 | 1.4 | [79] |
| Metal-Doped IONPs | ||||||
| Cu4-NP | 16.1 | 3.5 | 15.7 | 32.8 | 1.5 | [99] |
| EuIO-14 nanocubes | 14.0 ± 1.9 | 14.0 ± 1.9 | 36.79 ± 1.16 | 97.52 ± 2.16 | 0.5 | [100] |
| ZnFe2O4 | - | 4 | 7.93 | 14.64 | 1.5 | [101] |
| NiFe2O4 | - | 5 | 6.85 | 12.92 | 1.5 | [101] |
| Zn0.3Fe2.7O4@SiO2 | - | 18 | 615 | 1657 | 0.13 × 10−3 | [102] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Barahona, I.; Muñoz-Hernando, M.; Ruiz-Cabello, J.; Herranz, F.; Pellico, J. Iron Oxide Nanoparticles: An Alternative for Positive Contrast in Magnetic Resonance Imaging. Inorganics 2020, 8, 28. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics8040028
Fernández-Barahona I, Muñoz-Hernando M, Ruiz-Cabello J, Herranz F, Pellico J. Iron Oxide Nanoparticles: An Alternative for Positive Contrast in Magnetic Resonance Imaging. Inorganics. 2020; 8(4):28. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics8040028
Chicago/Turabian StyleFernández-Barahona, Irene, María Muñoz-Hernando, Jesus Ruiz-Cabello, Fernando Herranz, and Juan Pellico. 2020. "Iron Oxide Nanoparticles: An Alternative for Positive Contrast in Magnetic Resonance Imaging" Inorganics 8, no. 4: 28. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics8040028







