Chemistry of Phosphorene: Synthesis, Functionalization and Biomedical Applications in an Update Review
Abstract
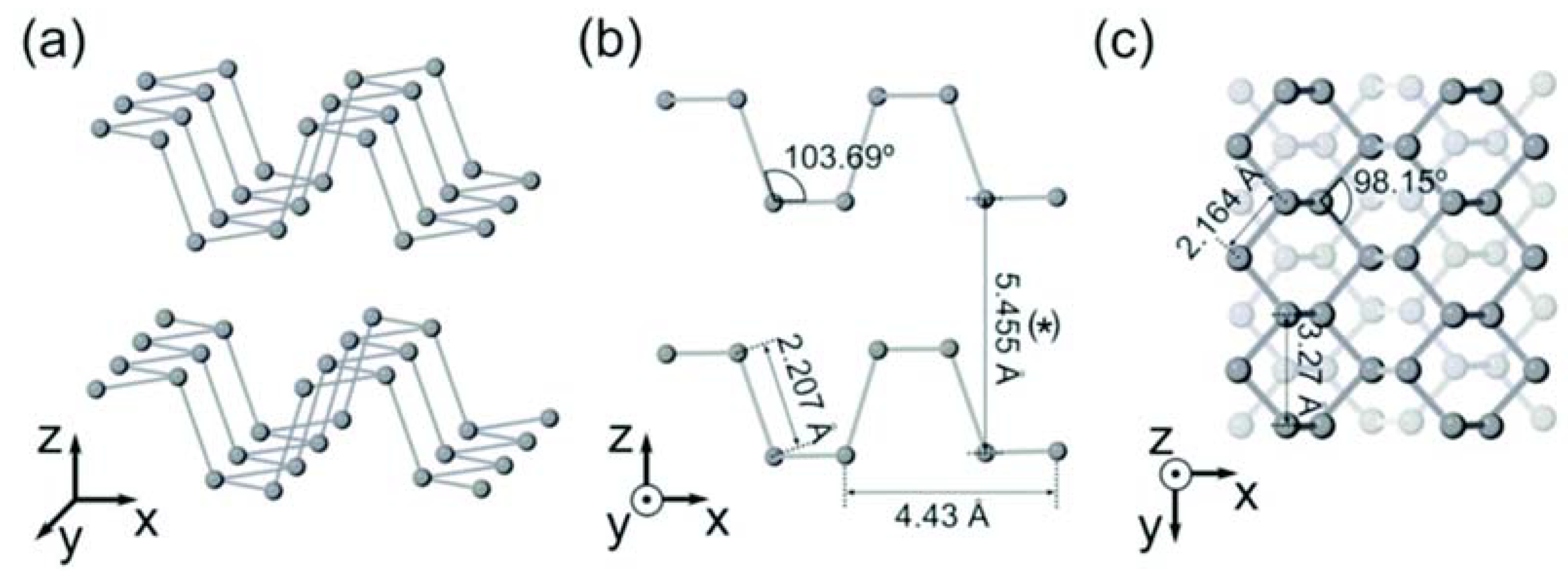
:1. Introduction
2. Preparation of Phosphorene
2.1. Top-Down Methods
2.1.1. Mechanical Exfoliation
2.1.2. Liquid Exfoliation
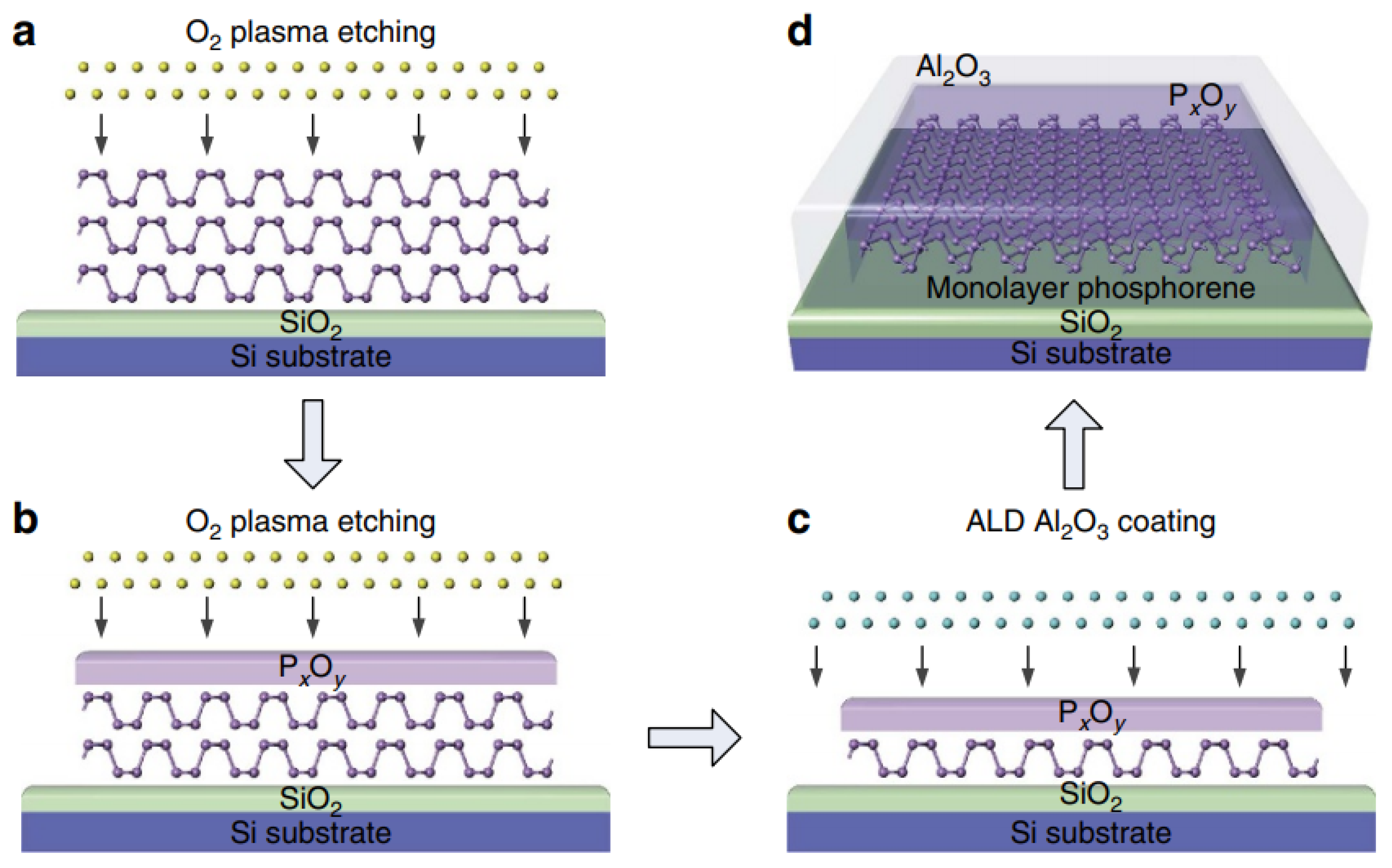
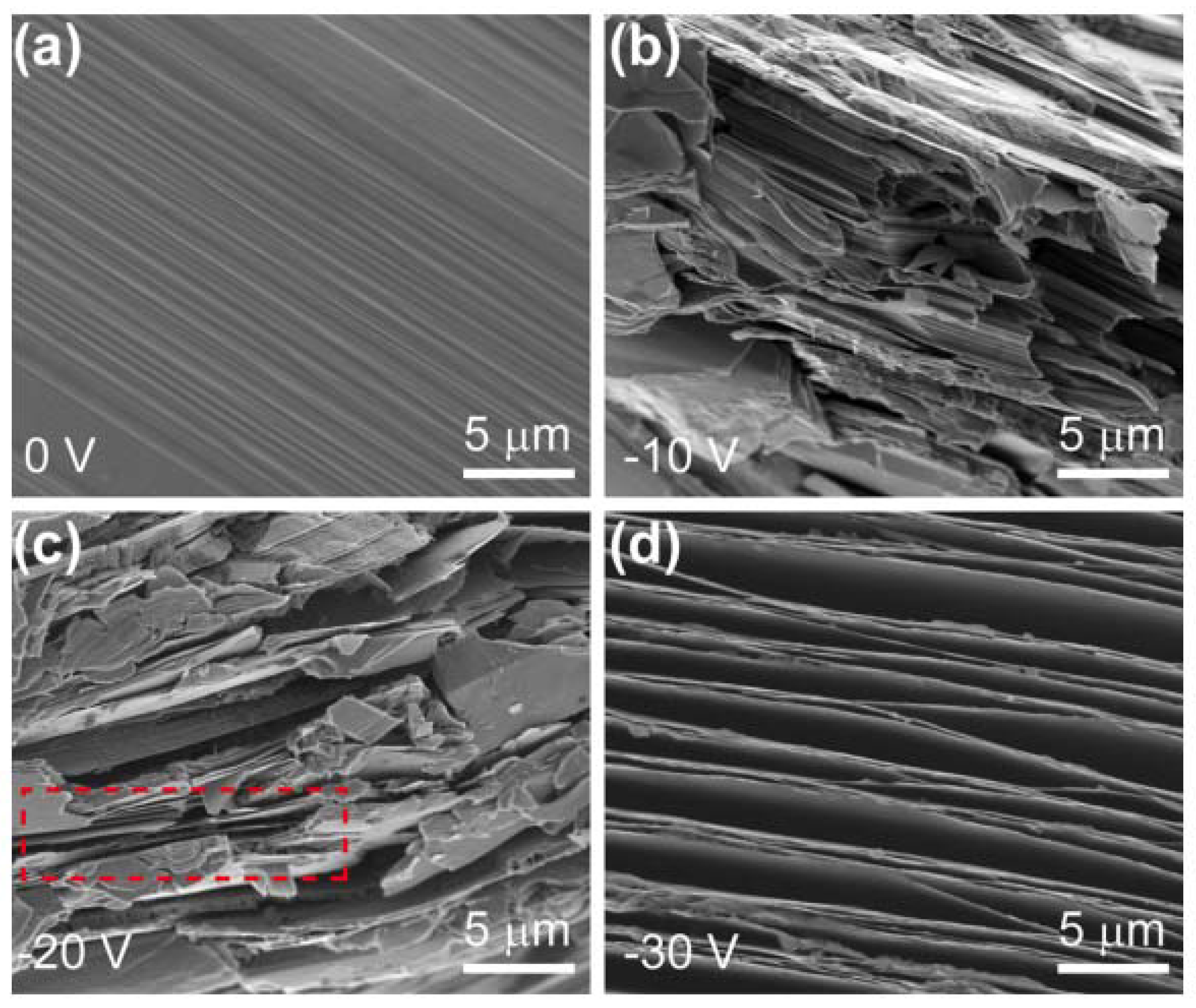
2.1.3. Electrochemical Exfoliation
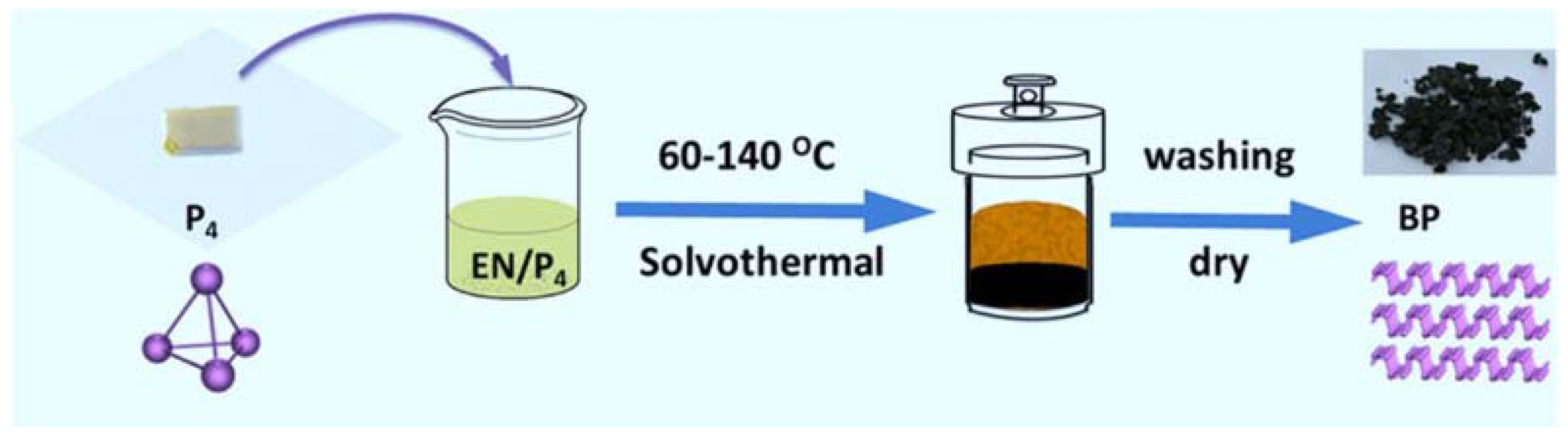
2.2. Bottom-Up Methods
3. Functionalization of Phosphorene
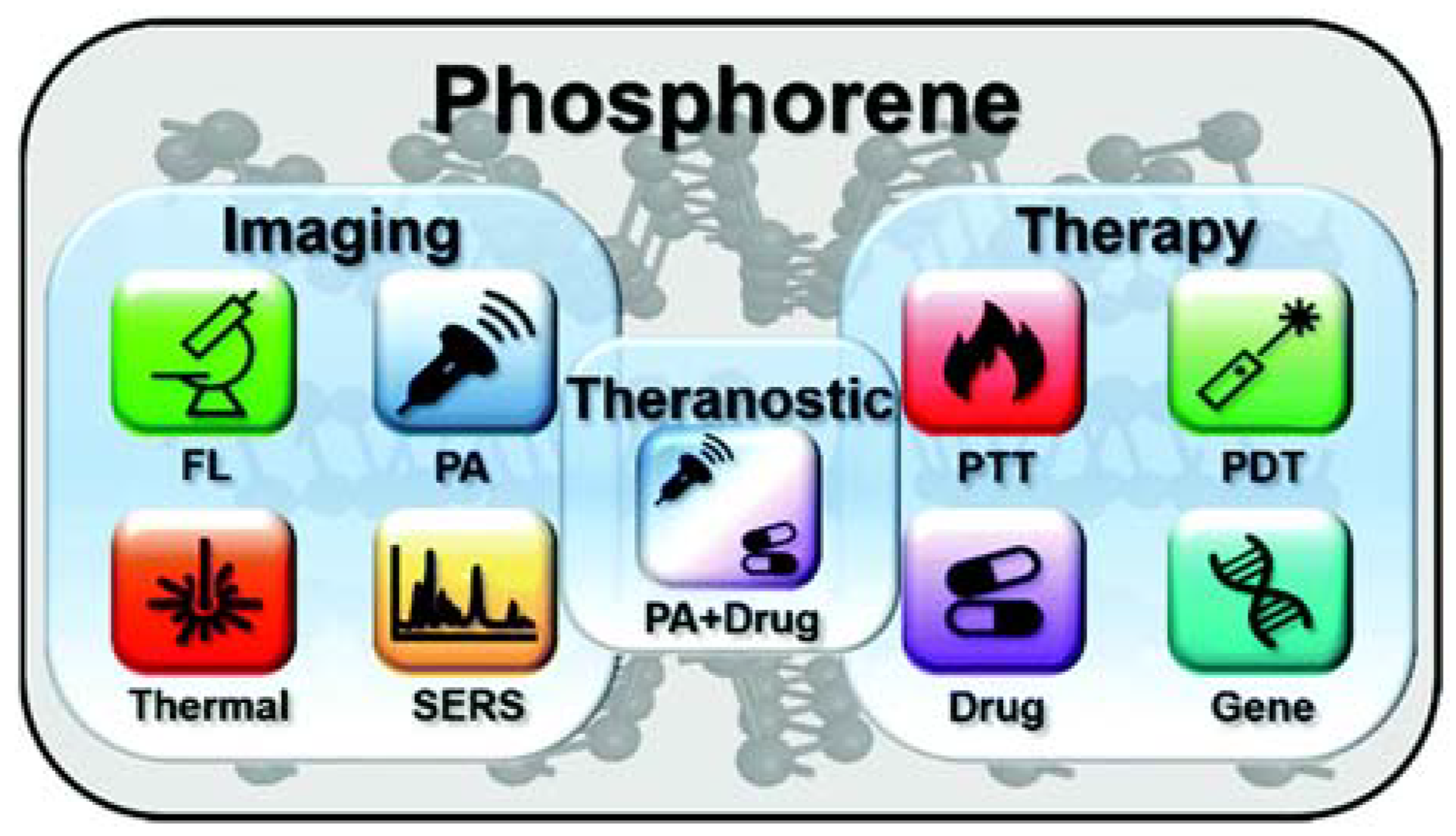
4. Biomedical Applications
5. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Kou, L.; Chen, C.; Smith, S.C. Phosphorene: Fabrication, properties, and applications. J. Phys. Chem. Lett. 2015, 6, 2794–2805. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, S.; Mansouri, N.; Aghaie, E. Phosphorene: A new competitor for graphene. Int. J. Hydrogen Energy 2016, 41, 4085–4095. [Google Scholar] [CrossRef]
- Batmunkh, M.; Bat-Erdene, M.; Shapter, J.G. Phosphorene and phosphorene-based materials—Prospects for future applications. Adv. Mater. 2016, 28, 8586–8617. [Google Scholar] [CrossRef]
- Liu, H.; Neal, A.T.; Zhu, Z.; Luo, Z.; Xu, X.; Tomanek, D.; Ye, P.D. Phosphorene: An unexplored 2D semiconductor with a high hole mobility. ACS Nano 2014, 8, 4033–4041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balendhran, S.; Walia, S.; Nili, H.; Sriram, S.; Bhaskaran, M. Elemental analogues of graphene: Silicene, germanene, stanene, and phosphorene. Small 2015, 11, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.B.; Hagaman, D.; Ji, H.-F. Growth of 2D black phosphorus film from chemical vapor deposition. Nanotechnology 2016, 27, 215602–215610. [Google Scholar] [CrossRef]
- Chaves, A.; Ji, W.; Maassen, J.; Dumitrică, T.; Low, T. Theoretical overview of black phosphorus. In 2D Materials: Properties and Devices; Avouris, P., Heinz, T., Low, T., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 381–412. [Google Scholar]
- Xu, Y.; Shi, Z.; Shi, X.; Zhang, K.; Zhang, H. Recent progress in black phosphorus and black-phosphorus-analogue materials: Properties, synthesis and applications. Nanoscale 2019, 11, 14491. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Wang, M.; Zhu, X.; Rodin, A.S.; Su, H.; Neto, A.H.C. Phosphorene: From theory to applications. Nat. Rev. Mater. 2016, 1, 16061–16069. [Google Scholar] [CrossRef]
- Sorkin, V.; Cai, Y.; Ong, Z.; Zhang, G.; Zhang, Y.W. Recent advances in the study of phosphorene and its nanostructures. Crit. Rev. Solid State Mater. Sci. 2017, 42, 1–82. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, G.; Zhang, Y.-W. (Eds.) Phosphorene: Physical Properties, Synthesis, and Fabrication; Jenny Stanford Publishing Pte. Ltd.: Singapore, 2019. [Google Scholar]
- Goswami, A.; Gawande, M.B. Phosphorene: Current status, challenges and opportunities. Front. Chem. Sci. Eng. 2019, 13, 296–309. [Google Scholar] [CrossRef]
- Bridgman, P.W. Two new modifications of Phosphorus. J. Am. Chem. Soc. 1914, 36, 1344–1363. [Google Scholar] [CrossRef] [Green Version]
- Hultgren, R.; Gingrich, N.S.; Warren, B.E. The atomic distribution in red and black phosphorus and the crystal structure of black phosphorus. J. Chem. Phys. 1935, 3, 351–355. [Google Scholar] [CrossRef]
- Brown, A.; Rundqvist, S. Refinement of the crystal structure of black phosphorus. Acta Cryst. 1965, 19, 684–685. [Google Scholar] [CrossRef]
- Akhtar, M.; Anderson, G.; Zhao, R.; Alruqi, A.; Mroczkowska, J.E.; Sumanasekera, G.; Jasinsk, J.B. Recent advances in synthesis, properties, and applications of phosphorene. NPJ 2D Mater. Appl. 2017, 1, 5. [Google Scholar] [CrossRef]
- Hu, Z.; Niu, T.; Guo, R.; Zhang, J.; Lai, M.; He, J.; Wang, L.; Chen, W. Two-dimensional black phosphorus: Its fabrication, functionalization and applications. Nanoscale 2018, 10, 21575–21603. [Google Scholar] [CrossRef]
- Martini, F.; Borsacchi, S.; Barcaro, G.; Caporali, M.; Vanni, M.; Serrano-Ruiz, M.; Geppi, M.; Peruzzini, M.; Calucci, L. Phosphorene and black phosphorus: The 31P NMR view. J. Phys. Chem. Lett. 2019, 10, 5122–5127. [Google Scholar] [CrossRef]
- Guan, L.; Xing, B.; Niu, X.; Wang, D.; Yu, Y.; Zhang, S.; Yan, X.; Wang, Y.; Sha, J. Metal-assisted exfoliation of few-layer black phosphorus with high yield. Chem. Commun. 2018, 54, 595–598. [Google Scholar] [CrossRef]
- Han, Z.J.; Murdock, A.T.; Seo, D.H.; Bendavid, A. Recent progress in plasma-assisted synthesis and modification of 2D materials. 2D Mater. 2018, 5, 32002–32028. [Google Scholar] [CrossRef]
- Lu, W.; Nan, H.; Hong, J.; Chen, Y.; Zhu, C.; Liang, Z.; Ma, X.; Ni, Z.; Jin, C.; Zhang, Z. Lasma-assisted fabrication of monolayer phosphorene and its Raman characterization. Nano Res. 2014, 7, 853–859. [Google Scholar] [CrossRef] [Green Version]
- Pei, J.; Gai, X.; Yang, J.; Wang, X.; Yu, Z.; Choi, D.-Y.; Luther-Davies, B.; Lu, Y. Producing air-stable monolayers of phosphorene and their defect engineering. Nat. Commun. 2016, 7, 10450–10458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuriakose, S.; Ahmed, T.; Balendhran, S.; Collis, G.E.; Bansal, V.; Aharonovich, I.; Sriram, S.; Bhaskaran, M.; Walia, S. Effects of plasma-treatment on the electrical and optoelectronic properties of layered black phosphorus. Appl. Mater. Today 2018, 12, 244–249. [Google Scholar] [CrossRef]
- Huang, H.; Gao, M.; Kang, Y.; Li, J.; Wang, J.; Wu, L.; Chu, P.K.; Huang, Y.; Ibarra, M.R.; Yu, X.-F. Rapid and scalable production of high-quality phosphorene by plasma–liquid technology. Chem. Commun. 2020, 56, 221–224. [Google Scholar] [CrossRef]
- Yan, Z.; He, X.; She, L.; Sun, J.; Jiang, R.; Xu, H.; Shi, F.; Lei, Z.; Liu, Z.-H. Solvothermal-assisted liquid-phase exfoliation of large size and high quality black phosphorus. J. Mater. 2018, 4, 129–134. [Google Scholar] [CrossRef]
- Gómez-Pérez, J.; Kónya, Z.; Kukovecz, A. Acetone improves the topographical homogeneity of liquid phase exfoliated few-layer black phosphorus flakes. Nanotechnology 2018, 29, 365303–365313. [Google Scholar] [CrossRef] [PubMed]
- Dhanabalan, S.C.; Ponraj, J.S.; Guo, Z.; Li, S.; Bao, Q.; Zhang, H. Emerging trends in phosphorene fabrication towards next generation devices. Adv. Sci. 2017, 4, 1600305–1600337. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhu, Y.; Chen, Z.; Lei, J.; Feng, P. Multilayer black phosphorus exfoliated with the aid of sodium hydroxide: An improvement in electrochemical energy storage. J. Electron. Mater. 2018, 47, 4793–4798. [Google Scholar] [CrossRef]
- Watts, M.C.; Picco, L.; Russell-Pavier, F.S.; Cullen, P.L.; Miller, T.S.; Bartuś, S.P.; Payton, O.D.; Skipper, N.T.; Tileli, V.; Howard, C.A. Production of phosphorene nanoribbons. Nature 2019, 568, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Sofer, Z.; Pumera, M. Electrochemical exfoliation of layered black phosphorus into phosphorene. Angew. Chem. Int. Ed. 2017, 56, 10443–10445. [Google Scholar] [CrossRef]
- Liu, N.; Kim, P.; Kim, J.H.; Ye, J.H.; Kim, S.; Lee, C.J. Large-area atomically thin MoS2 nanosheets prepared using electrochemical exfoliation. ACS Nano 2014, 8, 6902–6910. [Google Scholar] [CrossRef]
- Zhao, M.; Guo, X.Y.; Ambacher, O.; Nebel, C.E.; Hoffmann, R. Electrochemical generation of hydrogenated graphene flakes. Carbon 2015, 83, 128–135. [Google Scholar] [CrossRef]
- Wang, J.; Manga, K.K.; Bao, Q.; Loh, K.P. High-yield synthesis of few-layer graphene flakes through electrochemical expansion of graphite in propylene carbonate electrolyte. J. Am. Chem. Soc. 2011, 133, 8888–8891. [Google Scholar] [CrossRef]
- Xiao, H.; Zhao, M.; Zhang, J.; Ma, X.; Zhang, J.; Hu, T.; Tang, T.; Jia, J.; Wu, H. Electrochemical cathode exfoliation of bulky black phosphorus into few-layer phosphorene nanosheets. Electrochem. Commun. 2018, 89, 10–13. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Liu, S.; Lu, J.; Goh, W.P.; Fang, H.; Qiu, Z.; Tian, B.; Chen, Z.; Yao, C.; et al. Ultrafast electrochemical expansion of black phosphorus toward high-yield synthesis of few-layer phosphorene. Chem. Mater. 2018, 30, 2742–2749. [Google Scholar] [CrossRef]
- Luo, F.; Wang, D.; Zhang, J.; Li, X.; Liu, D.; Li, H.; Lu, M.; Xie, X.; Huang, L.; Haung, W. Ultrafast cathodic exfoliation of few-layer black phosphorus in aqueous solution. ACS Appl. Nano Mater. 2019, 2, 3793–3801. [Google Scholar] [CrossRef]
- Liu, H.; Lian, P.; Zhang, Q.; Yang, Y.; Mei, Y. The preparation of holey phosphorene by electrochemical assistance. Electrochem. Commun. 2019, 98, 124–128. [Google Scholar] [CrossRef]
- Khandelwal, A.; Mani, K.; Karigerasi, M.H.; Lahiri, I. Phophorene—The two dimensional black phosphorous: Properties, synthesis and applictions. Mater. Sci. Eng. B 2017, 221, 17–34. [Google Scholar] [CrossRef]
- Zhang, J.L.; Zhao, S.; Han, C.; Wang, Z.; Zhong, S.; Sun, S.; Guo, R.; Zhou, X.; Gu, C.D.; Yuan, K.D.; et al. Epitaxial growth of single layer blue phosphorus: A new phase of two-dimensional phosphorus. Nano Lett. 2016, 168, 4903–4908. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, G.; Zhang, Y.-W. The critical role of substrate in stabilizing phosphorene nanoflake: A theoretical exploration. J. Am. Chem. Soc. 2016, 138, 4763–4771. [Google Scholar] [CrossRef] [Green Version]
- Tian, B.; Tian, B.; Smith, B.; Scott, M.C.; Lei, Q.; Hua, R.; Tian, Y.; Liu, Y. Facile bottom-up synthesis of partially oxidized black phosphorus nanoshets as metal-free photocatalyst for hydrogen evolution. Proc. Natl. Acad. Sci. USA 2018, 115, 4345–4350. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Han, X.; Li, Z.; Liu, W.; Li, X.; Wu, J.; Guo, Z.; Liu, H. Epitaxial growth of few-layer black phosphorene quantum dots on Si substrates. Adv. Mater. Interfaces 2018, 5, 1801048–1801056. [Google Scholar] [CrossRef]
- Sang, D.K.; Wang, H.; Guo, Z.; Xie, N.; Zhang, H. Recent developments in stability and passivation techniques of phosphorene toward next-generation device applications. Adv. Funct. Mater. 2019, 29, 1903419–1903431. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, Q.; Tong, Y.; Wang, J. Light-induced ambient degradation of Fe-layer black phosphorus: Mechanism and protection. Angew. Chem. Int. Ed. 2016, 55, 11437–11441. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Singh, Y.; Cho, S.-Y.; Sasikala, S.P.; Koo, S.H.; Narayan, R.; Jung, H.-T.; Jung, Y.; Kim, S.O. Ambient stabilization of few layer phosphorene via noncovalent functionalization with surfactants: Systematic 2D NMR characterization in aqueous dispersion. Chem. Mater. 2019, 31, 2786–2794. [Google Scholar] [CrossRef]
- Gusmão, R.; Sofer, Z.; Pumera, M. Functional protection of exfoliated black phosphorus by noncovalent modification with anthraquinone. ACS Nano 2018, 12, 5666–5673. [Google Scholar] [CrossRef] [PubMed]
- Ghambarian, M.; Azizi, Z.; Ghashghaee, M. Functionalization and doping of black phosphorus. In Black Phosphorus; Inamuddin, I., Boddula, R., Asiri, A., Eds.; Engineering Materials; Springer: Cham, Switzerland, 2020; pp. 1–30. [Google Scholar]
- Kumar, A. Simultaneous passivation and encapsulation of black phosphorus nanosheets (phosphorene) by optically active polypeptide micelles for biosensors. ACS Appl. Nano Mater. 2019, 2, 2397–2404. [Google Scholar] [CrossRef]
- Liang, S.; Wu, L.; Liu, H.; Li, J.; Chen, M.; Zhang, M. Organic molecular passivation of phosporene: An aptamer-based biosensing platform. Biosens. Bioelectron. 2019, 126, 30–35. [Google Scholar] [CrossRef]
- Bolognesi, M.; Moschetto, S.; Trapani, M.; Prescimone, F.; Ferroni, C.; Manca, G.; Ienco, A.; Borsacchi, S.; Caporali, M.; Muccini, M.; et al. Noncovalent functionalization of 2D black phosphorus with fluorescent boronic derivatives of pyrene for probing and modulating the interaction with molecular oxygen. ACS Appl. Mater. Interfaces 2019, 11, 22637–22647. [Google Scholar] [CrossRef]
- Qiu, M.; Ren, W.X.; Jeong, T.; Won, M.; Park, G.Y.; Sang, D.K.; Liu, L.-P.; Zhang, H.; Kim, J.S. Omnipotent phosphorene: A next-generation, two-dimensional nanoplatform for multidisciplinary biomedical applications. Chem. Soc. Rev. 2018, 47, 5588–5601. [Google Scholar] [CrossRef]
- Luo, M.; Fan, T.; Zhou, Y.; Zhang, H.; Mei, L. 2D black phosphorus-based biomedical applications. Adv. Funct. Mater. 2019, 29, 1808306–1808325. [Google Scholar] [CrossRef]
- Tatullo, M.; Genovese, F.; Aiello, E.; Amantea, M.; Makeeva, I.; Zavan, B.; Rengo, S.; Fortunato, L. Phosphorene is the new graphene in biomedical applications. Materials 2019, 12, 2301. [Google Scholar] [CrossRef] [Green Version]
- Anju, S.; Ashtami, J.; Mohanan, P.V. Black phosphorus, a prospective graphene substitute for biomedical applications. Mater. Sci. Eng. C 2019, 97, 978–993. [Google Scholar]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Nilghaz, A.; Lin, Y.; Xu, J.; Lu, X. Black phosphorus and its biomedical applications. Theranostics 2018, 8, 1005–1026. [Google Scholar] [CrossRef] [PubMed]
- Qu, G.; Xia, T.; Zhou, W.; Zhang, X.; Zhang, H.; Hu, L.; Shi, J.; Yu, X.-F.; Jiang, G. Property-activity relationship of black phosphorus at the nano–bio interface: From molecules to organisms. Chem. Rev. 2020, 120, 2288–2346. [Google Scholar]
- Sun, Z.; Zhang, Y.; Yu, H.; Yan, C.; Liu, Y.; Hong, S.; Tao, H.; Robertson, A.W.; Wang, Z.; Pádua, A.A.H. New solvent-stabilized few-layer black phosphorus for antibacterial applications. Nanoscale 2018, 10, 12543–12553. [Google Scholar] [CrossRef] [PubMed]
- Mayorga-Martinez, C.C.; Gusmão, R.; Sofer, Z.; Pumera, M. Pnictogen-based enzymatic phenol biosensors: Phosphorene, arsenene, antimonene, and bismuthene. Angew. Chem. Int. Ed. 2019, 58, 134–138. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Sofer, Z.; Pumera, M. Binary phosphorene redox behavior in oxidoreductase enzymatic systems. ACS Nano 2019, 13, 13217–13224. [Google Scholar] [PubMed]
- Liang, H.; Xu, H.; Zhao, Y.; Zheng, J.; Zhao, H.; Li, G.; Li, C.-P. Ultrasensitive electrochemical sensor for prostate specific antigen detection with a phosphorene platform and magnetic covalent organic framework signal amplifier. Biosens. Bioelectron. 2019, 144, 111691. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, W.; Liu, D.; Huang, H.; Dan, K.; Hu, X.; Yu, S.; Chu, P.K.; Yu, X.-F. Template growth of Au/Ag nanocomposites on phosphorene for sensitive SERS detection of pesticides. Nanotechnology 2019, 30, 275604–275612. [Google Scholar] [CrossRef]
- Comber, S.; Gardner, M.; Georges, K.; Blackwood, D.; Gilmour, D. Domestic source of phosphorus to sewage treatment works. Environ. Technol. 2013, 34, 1349–1358. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Wu, J.; Gu, Z. Black phosphorus hydrogel scaffolds enhance bone regeneration via a sustained supply of calcium-free phosphorus. ACS Appl. Mater. Interfaces 2018, 11, 2908–2916. [Google Scholar]
- Shao, J.; Ruan, C.; Xie, H.; Li, Z.; Wang, H.; Chu, P.K.; Yu, X.-F. Black-phosphorus-incorporated hydrogel as a sprayable and biodegradable photothermal platform for postsurgical treatment of cancer. Adv. Sci. 2018, 5, 1700848–1700855. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Tsai, H.-I.; Zeng, X.; Qi, J.; Luo, M.; Wang, X.; Mei, L.; Deng, W. Black phosphorus nanosheets-based stable drug delivery system via drug-self-stabilization for combined photothermal and chemo cancer therapy. Chem. Eng. J. 2019, 375, 121917–121927. [Google Scholar] [CrossRef]
- Zong, S.; Wang, L.; Yang, Z.; Wang, H.; Wang, Z.; Cui, Y. Black phosphorus-based drug nanocarrier for targeted and synergetic chemophotothermal therapy of acute lymphoblastic leukemia. ACS Appl. Mater. Interfaces 2019, 11, 5896–5902. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pica, M.; D’Amato, R. Chemistry of Phosphorene: Synthesis, Functionalization and Biomedical Applications in an Update Review. Inorganics 2020, 8, 29. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics8040029
Pica M, D’Amato R. Chemistry of Phosphorene: Synthesis, Functionalization and Biomedical Applications in an Update Review. Inorganics. 2020; 8(4):29. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics8040029
Chicago/Turabian StylePica, Monica, and Roberto D’Amato. 2020. "Chemistry of Phosphorene: Synthesis, Functionalization and Biomedical Applications in an Update Review" Inorganics 8, no. 4: 29. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics8040029




