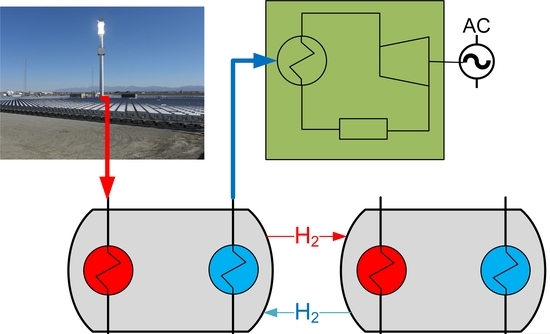
Techno-Economic Assessment of Destabilized Li Hydride Systems for High Temperature Thermal Energy Storage
Abstract
:1. Introduction
2. Results
2.1. Baseline Thermal Energy Storage System Results
2.2. Sensitivity Analysis Results
3. Thermal Energy Storage Systems with Destabilized Lithium Hydrides
3.1. Destabilized Li Materials for Thermal Energy Storage Applications
3.2. Li-Based Thermal Energy Storage System Characteristics
4. Techno-Economic Analysis
4.1. Techno-Economic Analysis Model
4.2. Assumed Degrees of Freedom
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
Nomenclature and Abbreviations
| A | Heat transfer area (m2) |
| C | Installed cost ($) |
| Cost | Specific cost ($·kWhth−1) |
| CSP | Concentrating solar power |
| D | Heat exchanger tube diameter (m) |
| ENG | Expanded natural graphite |
| f | Cost installation factor for the heat exchanger and pressure vessel |
| fm | Metal hydride material cost factor |
| FOB | Free on board |
| HE&PV | Heat exchanger and pressure vessel |
| heff | Overall heat transfer coefficient (W·m−2·K−1) |
| HT | High temperature |
| HTMH | High temperature metal hydride |
| k | Thermal conductivity (W·m−1·K−1) |
| L | Heat exchanger tube length (m) |
| LMTD | Log mean temperature difference (°C) |
| LT | Low temperature |
| LTMH | Low temperature metal hydride |
| M | Mass (kg) |
| MH | Metal hydride |
| PCF | Plant capacity factor |
| PP | Power plant |
| SAH | Sodium aluminum hydride |
| sCO2 | Supercritical CO2 |
| TES | Thermal energy storage |
| Wel | Power plant electric power (W or kW) |
| wt | Metal hydride hydrogen weight fraction (kgH2·kgMH−1) |
| Wth | Thermal power (W or kW) |
| XRD | X ray diffraction |
| Greek symbols | |
| ∆H | Metal hydride reaction enthalpy (kJ·molH2−1) |
| ∆S | Metal hydride reaction entropy (kJ·molH2−1·K−1) |
| ∆ts | Storage time (h) |
| ∆T | Temperature difference (°C) |
| ηPP | Power plant energy efficiency (%) |
| ρ | Material bulk density (kg·m−3) |
References
- Denholm, P.; Mehos, M. Enabling greater penetration of solar power via the use of CSP with thermal energy storage. In Solar Energy: Application, Economics, and Public Perception; Adamarola, M., Ed.; Apple Academic Press: Oakville, ON, Canada; CRC Press: Boca Raton, FL, USA, 2014; p. 99. [Google Scholar]
- Weinstein, L.A.; Loomis, J.; Bhatia, B.; Bierman, D.M.; Wang, E.N.; Chen, G. Concentrating solar power. Chem. Rev. 2015, 115, 12797–12838. [Google Scholar] [CrossRef] [PubMed]
- Denholm, P.; Hand, M. Grid Flexibility and Storage Required to Achieve Very High Penetration of Variable Renewable Electricity. Energy Policy 2011, 39, 1817–1830. [Google Scholar] [CrossRef]
- Gil, A.; Medrano, M.; Martorell, I.; Lázaro, A.; Dolado, P.; Zalba, B.; Cabeza, L.F. State of the art on high temperature thermal energy storage for power generation. Part 1—Concepts, materials and modellization. Renew. Sustain. Energy Rev. 2010, 14, 31–55. [Google Scholar] [CrossRef]
- Alva, G.; Liu, L.; Huang, X.; Fang, G. Thermal energy storage materials and systems for solar energy applications. Renew. Sustain. Energy Rev. 2017, 68, 693–706. [Google Scholar] [CrossRef]
- Pelay, U.; Luo, L.; Fan, Y.; Stitou, D.; Rood, M. Thermal energy storage systems for concentrated solar power plants. Renew. Sustain. Energy Rev. 2017, 79, 82–100. [Google Scholar] [CrossRef]
- Sarbu, I.; Sebarchievici, C. A comprehensive review of thermal energy storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Kuravi, S.; Trahan, J.; Goswami, Y.; Rahman, M.M.; Stefanakos, E.K. Thermal energy storage technologies and systems for concentrating solar power plants. Prog. Energy Combust. Sci. 2013, 39, 285–319. [Google Scholar] [CrossRef]
- Sheppard, D.A.; Buckley, C.E. The potential of metal hydrides paired with compressed hydrogen as thermal energy storage for concentrating solar power plants. Int. J. Hydrog. Energy 2019, 44, 9143–9163. [Google Scholar] [CrossRef]
- Corgnale, C.; Hardy, B.; Motyka, T.; Zidan, R.; Teprovich, J.; Peters, B. Screening analysis of metal hydride based thermal energy storage systems for concentrating solar power plants. Renew. Sustain. Energy Rev. 2014, 38, 821–833. [Google Scholar] [CrossRef]
- Reiser, A.; Bogdanovic, B.; Schlichte, K. The application of Mg-based metal-hydrides as heat energy storage systems. Int. J. Hydrog. Energy 2000, 25, 425–430. [Google Scholar] [CrossRef]
- Bogdanovic, B.; Hofmann, H.; Neuy, A.; Reiser, A.; Schlichte, K. Ni-doped versus undoped Mg–MgH2 materials for high temperature heat or hydrogen storage. J. Alloys Compd. 1999, 292, 57–71. [Google Scholar] [CrossRef]
- d’Entremont, A.; Corgnale, C.; Sulic, M.; Hardy, B.; Zidan, R.; Motyka, T. Modeling of a thermal energy storage system based on coupled metal hydrides (magnesium iron–sodium alanate) for concentrating solar power plants. Int. J. Hydrog. Energy 2017, 42, 22518–22529. [Google Scholar] [CrossRef]
- Sheppard, D.; Paskevicius, M.; Buckley, C. Thermodynamics of hydrogen desorption from NaMgH3 and its application as a solar heat storage medium. Chem. Mater. 2011, 23, 4298–4300. [Google Scholar] [CrossRef]
- Sheppard, D.A.; Corgnale, C.; Hardy, B.; Motyka, T.; Zidan, R.; Paskevicius, M.; Buckley, C.E. Hydriding characteristics of NaMgH2F with preliminary technical and cost evaluation of magnesium-based metal hydride materials for concentrating solar power thermal storage. RSC Adv. 2014, 4, 26552–26562. [Google Scholar] [CrossRef]
- d’Entremont, A.; Corgnale, C.; Hardy, B.; Zidan, R. Simulation of high temperature thermal energy storage system based on coupled metal hydrides for solar driven steam power plants. Int. J. Hydrog. Energy 2018, 43, 817–830. [Google Scholar] [CrossRef]
- Ward, P.A.; Corgnale, C.; Teprovich, J.A., Jr.; Motyka, T.; Hardy, B.; Peters, B.; Zidan, R. High performance metal hydride based thermal energy storage systems for concentrating solar power applications. J. Alloys Compd. 2015, 645, S374–S378. [Google Scholar] [CrossRef] [Green Version]
- Rönnebro, E.C.; Whyatt, G.; Powell, M.; Westman, M.; Zheng, F.R.; Fang, Z.Z. Metal hydrides for high-temperature power generation. Energies 2015, 8, 8406–8430. [Google Scholar] [CrossRef] [Green Version]
- Caldwell, R.T.; McDonald, J.W.; Pietsch, A. Solar-energy receiver with lithium-hydride heat storage. Sol. Energy 1965, 9, 48–60. [Google Scholar] [CrossRef]
- Hanold, R.J.; Johnston, R.D. Power Plant Heat Storage Arrangement. US Patent US3029596, 14 April 1962. [Google Scholar]
- US Department of Energy SunShot Vision Study. Available online: https://www.energy.gov/sites/prod/files/SunShot%20Vision%20Study.pdf (accessed on 15 February 2020).
- Ward, P.A.; Teprovich, J.A.; Liu, Y.; He, J.; Zidan, R. High temperature thermal energy storage in the CaAl2 system. J. Alloys Compd. 2018, 735, 2611–2615. [Google Scholar] [CrossRef]
- Javadian, P.; Sheppard, D.A.; Jensen, T.R.; Buckley, C.E. Destabilization of lithium hydride and the thermodynamic assessment of the Li–Al–H system for solar thermal energy storage. RSC Adv. 2016, 6, 94927–94933. [Google Scholar] [CrossRef]
- Corgnale, C.; Hardy, B.; Motyka, T.; Zidan, R. Metal hydride based thermal energy storage system requirements for high performance concentrating solar power plants. Int. J. Hydrog. Energy 2016, 41, 20217–20230. [Google Scholar] [CrossRef] [Green Version]
- Sangster, J.; Pelton, A.D. H–Li (Hydrogen–Lithium). In Phase Diagrams of Binary Hydrogen Alloys; Manchester, F.D., Ed.; ASM International: Cleveland, OH, USA, 2000; pp. 74–81. [Google Scholar]
- Yu, X.B.; Grant, D.M.; Walker, G.S. A new dehydrogenation mechanism for reversible multicomponent borohydride systems—The role of Li–Mg alloys. Chem. Commun. 2006, 37, 3906–3908. [Google Scholar] [CrossRef] [PubMed]
- Vajo, J.J.; Skeith, S.L.; Mertens, F. Reversible storage of hydrogen in destabilized LiBH4. J. Phys. Chem. B 2005, 109, 3719–3722. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Hino, S.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. Hydrogen storage properties of lithium silicon alloy synthesized by mechanical alloying. J. Power Sources 2011, 196, 504–507. [Google Scholar] [CrossRef] [Green Version]
- Vajo, J.J.; Mertens, F.; Ahn, C.C.; Bowman, R.C.; Fultz, B. Altering hydrogen storage properties by hydride destabilization through alloy formation, LiH and MgH2 destabilized with Si. J. Phys. Chem. B 2004, 108, 13977–13983. [Google Scholar] [CrossRef]
- Jain, A.; Kawasako, E.; Miyaoka, H.; Ma, T.; Isobe, S.; Ichikawa, T.; Kojima, Y. Destabilization of LiH by Li insertion into Ge. J. Phys. Chem. C 2013, 117, 5650–5657. [Google Scholar] [CrossRef]
- Abbas, M.A.; Grant, D.M.; Brunelli, M.; Hansen, T.C.; Walker, G.S. Reducing the dehydrogenation temperature of lithium hydride through alloying with germanium. Phys. Chem. Chem. Phys. 2013, 15, 12139–12146. [Google Scholar] [CrossRef]
- Goward, G.R.; Taylor, N.J.; Souza, D.C.; Nazar, L.F. The true crystal structure of Li17M4 (M = Ge, Sn, Pb)–revised from Li22M5. J. Alloys Compd. 2001, 329, 82–91. [Google Scholar] [CrossRef]
- Lupu, C.; Mao, J.G.; Rabalais, J.W.; Guloy, A.M.; Richardson, J.W. X-ray and neutron diffraction studies on “Li4.4Sn”. Inorg. Chem. 2003, 42, 3765–3771. [Google Scholar] [CrossRef]
- Jain, A.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. Correlation between electrochemical behavior and hydrogen storage properties of Li–Sn system. J. Alloys Compd. 2013, 580, S211–S215. [Google Scholar] [CrossRef]
- Jain, A.; Miyaoka, H.; Ichikawa, T. Destabilization of lithium hydride by the substitution of group 14 elements, A review. Int. J. Hydrog. Energy 2016, 41, 5969–5978. [Google Scholar] [CrossRef]
- Corgnale, C.; Hardy, B.J.; Tamburello, D.A.; Garrison, S.L.; Anton, D.L. Acceptability envelope for metal hydride-based hydrogen storage systems. Int. J. Hydrog. Energy 2012, 37, 2812–2824. [Google Scholar] [CrossRef] [Green Version]
- Motyka, T. Hydrogen Storage Engineering Center of Excellence Metal Hydride Final Report; Savannah River Site (SRS), Savannah River National Lab.(SRNL): Aiken, SC, USA, 2014. [Google Scholar]
- Silicon Price. Available online: https://0-www-statista-com.brum.beds.ac.uk/statistics/301564/us-silicon-price-by-type/ (accessed on 11 February 2020).
- Germanium Price. Available online: https://0-www-statista-com.brum.beds.ac.uk/statistics/1061511/us-germanium-price/ (accessed on 11 February 2020).
- Tin Price. Available online: https://0-www-statista-com.brum.beds.ac.uk/statistics/675883/average-prices-tin-worldwide/ (accessed on 11 February 2020).
- Aluminum Price. Available online: https://0-www-statista-com.brum.beds.ac.uk/statistics/675845/average-prices-aluminum-worldwide/ (accessed on 30 January 2020).
- Veleckis, E. Thermodynamic investigation of the Li–Al and Li–Pb systems by the hydrogen titration method. J. Less Common Met. 1980, 73, 49–60. [Google Scholar] [CrossRef]
- Bogdanović, B.; Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials. J. Alloys Compd. 1997, 253, 1–9. [Google Scholar] [CrossRef]
- Ward, P.A.; Corgnale, C.; Teprovich, J.A.; Motyka, T.; Hardy, B.; Sheppard, D.; Buckley, C.; Zidan, R. Technical challenges and future direction for high-efficiency metal hydride thermal energy storage systems. Appl. Phys. A 2016, 122, 462. [Google Scholar] [CrossRef]
- Guthrie, K.M. Data and Techniques For Preliminary Capital Cost Estimating. Chem. Eng. Prog. 1969, 76, 114–142. [Google Scholar]
- Douglas, E. Industrial Chemical Process Design; McGraw-Hill Professional Engineering: New York, NY, USA, 2003. [Google Scholar]
- Aspen In Plant Cost Estimator. ASPEN Tech Manual. Available online: https://www.aspentech.com/en/products/pages/aspen-in-plant-cost-estimator (accessed on 18 February 2020).
- Cerri, G.; Salvini, C.; Corgnale, C.; Giovannelli, A.; Manzano, D.D.; Martinez, A.O.; Le Duigou, A.; Borgard, J.M.; Mansilla, C. Sulfur–Iodine plant for large scale hydrogen production by nuclear power. Int. J. Hydrog. Energy 2010, 35, 4002–4014. [Google Scholar] [CrossRef]
- Motyka, T. Savannah River National Laboratory Regenerative Fuel Cell Project; SRNL-STI-2008-00388; Savannah River National Lab.(SRNL): Aiken, SC, USA, 2008. [Google Scholar]
- Corgnale, C.; Summers, W.A. Solar hydrogen production by the Hybrid Sulfur process. Int. J. Hydrog. Energy 2011, 36, 11604–11619. [Google Scholar] [CrossRef]
- Corgnale, C.; Motyka, T.; Greenway, S.; Perez-Berrios, J.; Nakano, A.; Ito, I. Metal hydride bed system model for renewable source driven Regenerative Fuel Cell. J. Alloys Compd. 2013, 580, S406–S409. [Google Scholar] [CrossRef]
- Corgnale, C.; Sulic, M. Techno-Economic Analysis of High-Pressure Metal Hydride Compression Systems. Metals 2018, 8, 469. [Google Scholar] [CrossRef] [Green Version]
- Kolb, G.J.; Ho, C.K.; Mancini, T.R.; Gary, J.A. Power Tower Technology Roadmap and Cost Reduction Plreduction Plan; SAND2011-2419; Sandia National Laboratories: Albuquerque, NM, USA, 2011. [Google Scholar]
- Bayon, A.; Bader, R.; Jafarian, M.; Fedunik-Hofman, L.; Sun, Y.; Hinkley, J.; Miller, S.; Lipiński, W. Techno-economic assessment of solid–gas thermochemical energy storage systems for solar thermal power applications. Energy 2018, 149, 473–484. [Google Scholar] [CrossRef]

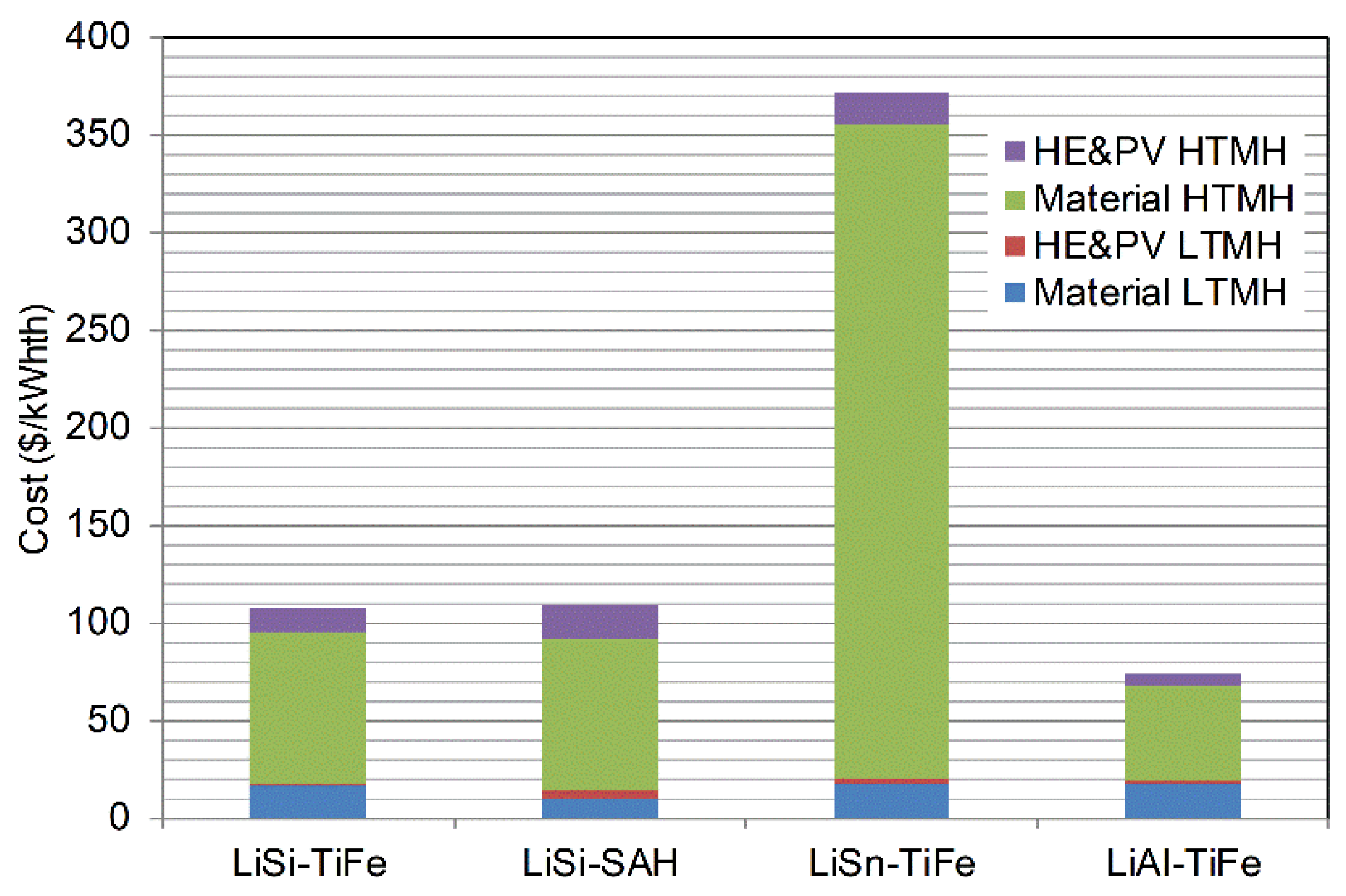
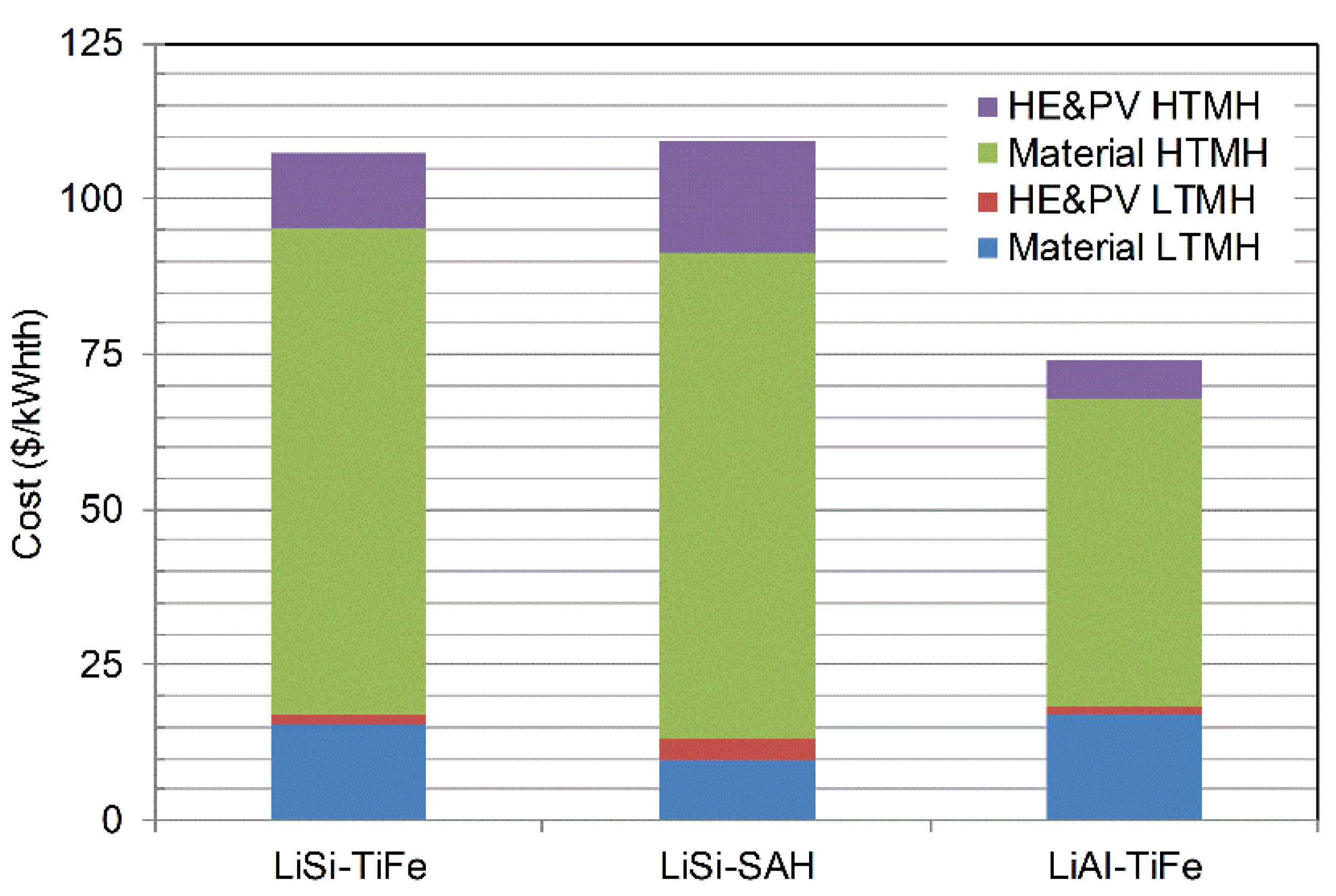



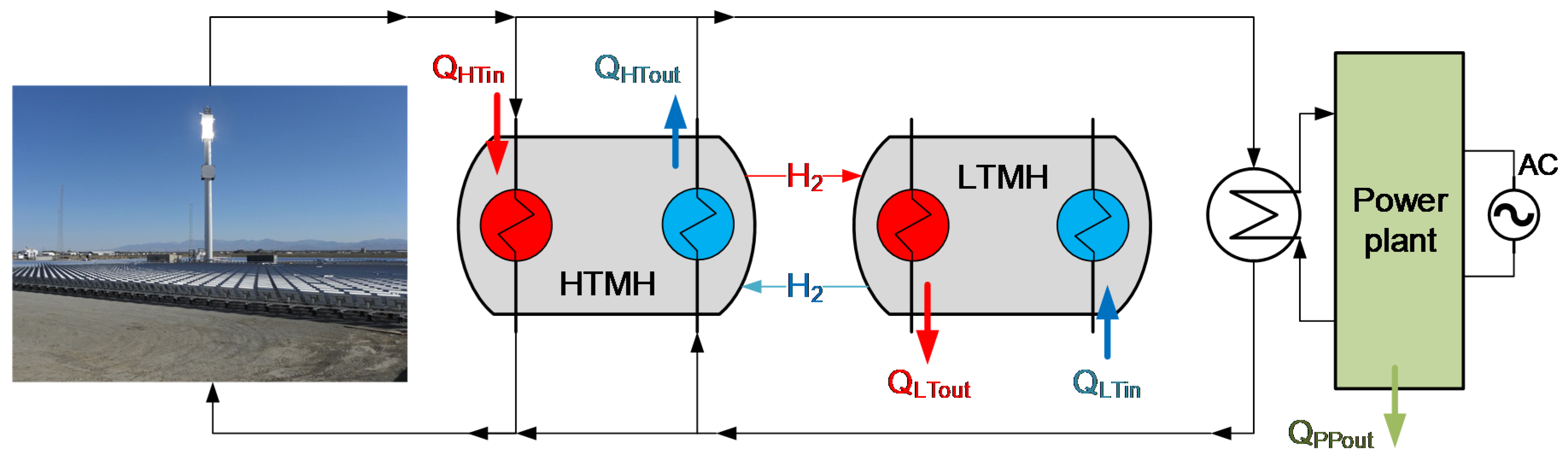
| TES | WthHTMH (MW) | WthLTMH (MW) | MH2 (ton) | MHTHM (ton) | MLTHM (ton) | AHTMH (m2) | ALTMH (m2) |
|---|---|---|---|---|---|---|---|
| LiSi-TiFe | 352.7 | 93.1 | 310 | 12,090 | 16,006 | 71,730 | 11,369 |
| LiSi-SAH | 317.5 | 140.1 | 279 | 10,881 | 10,881 | 64,557 | 18,271 |
| LiSn-TiFe | 317.5 | 95.9 | 320 | 45,325 | 16,497 | 55,810 | 11,718 |
| LiAl-TiFe | 352.7 | 100.9 | 336 | 13,673 | 17,359 | 41,451 | 12,330 |
| HTMH Material | wt % (100·kgH2·kgMH−1) | ρ * (kg·m−3) | k ** (W·m−1·K−1) | ∆H abs/des (kJ·molH2−1) | ∆S abs/des (J·molH2−1·K−1) | Raw MH Cost ($/kg) | Operating T (°C)/P (bar) | References |
|---|---|---|---|---|---|---|---|---|
| LiSi (Eqn 2) | 2.5 | 733 | 3.0 | 106.5/120.7 | 139.7/155 *** | 28.1 | 600–750/8.4–72.5 | [29,38] |
| LiGe (Eqn 4) | 0.9 | 1212 | 3.0 | 79.02 | 117.45 | 730.6 | 650/46 | [30,32,39] |
| LiSn (Eqn 5) | 0.7 | 1277 | 4.5 | 93.0 | 100.0 | 29.0 | 750/3 | [34,40] |
| LiAl (Eqn 7) | 2.4 | 1132 | 6.0 | 98.2 | 117.2 | 15.8 | 600/2 | [23,41,42] |
| HTMH Material | wt % (100·kgH2·kgMH−1) | ρ * (kg·m−3) | k ** (W·m−1·K−1) | ∆H abs/des (kJ·molH2−1) | ∆S abs/des (J·molH2−1·K−1) | Raw MH cost ($/kg) | Operating T (°C)/P (bar) | References |
|---|---|---|---|---|---|---|---|---|
| TiFe | 1.9 | 2500 | 7.0 | 28.1 | 106 | 4.0 | 0–100/1.4–40 | [10] |
| SAH (Na3AlH6) | 2.5 | 750 | 7.0 | 47 | 132 | 3.0 | 120–220/4.5–82 | [10,24,36,37] |
| TES Materials | Power Plant | Temperature (°C) | ηPP (%) | PCF | ∆ts (h) | Reference |
|---|---|---|---|---|---|---|
| LiSi-TiFe | Steam (100 MWel) | 600–650 | 45 | 63 | 13 | [10,21,44] |
| LiSi-SAH | sCO2 (100 MWel) | 700–750 | 50 | 63 | 13 | [10,21,44] |
| LiSn-TiFe | sCO2 (100 MWel) | 730–760 | 50 | 63 | 13 | [10,21,44] |
| LiAl-TiFe | Steam (100 MWel) | 550–600 | 45 | 63 | 13 | [10,21,44] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corgnale, C. Techno-Economic Assessment of Destabilized Li Hydride Systems for High Temperature Thermal Energy Storage. Inorganics 2020, 8, 30. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics8050030
Corgnale C. Techno-Economic Assessment of Destabilized Li Hydride Systems for High Temperature Thermal Energy Storage. Inorganics. 2020; 8(5):30. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics8050030
Chicago/Turabian StyleCorgnale, Claudio. 2020. "Techno-Economic Assessment of Destabilized Li Hydride Systems for High Temperature Thermal Energy Storage" Inorganics 8, no. 5: 30. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics8050030