Susceptibility of the Formate Hydrogenlyase Reaction to the Protonophore CCCP Depends on the Total Hydrogenase Composition
Abstract
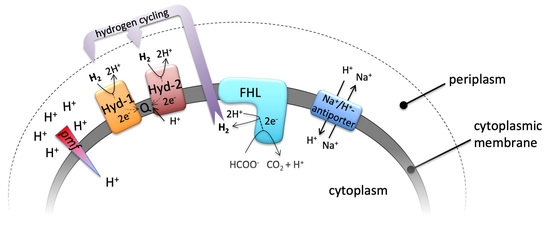
:1. Introduction
2. Results and Discussion
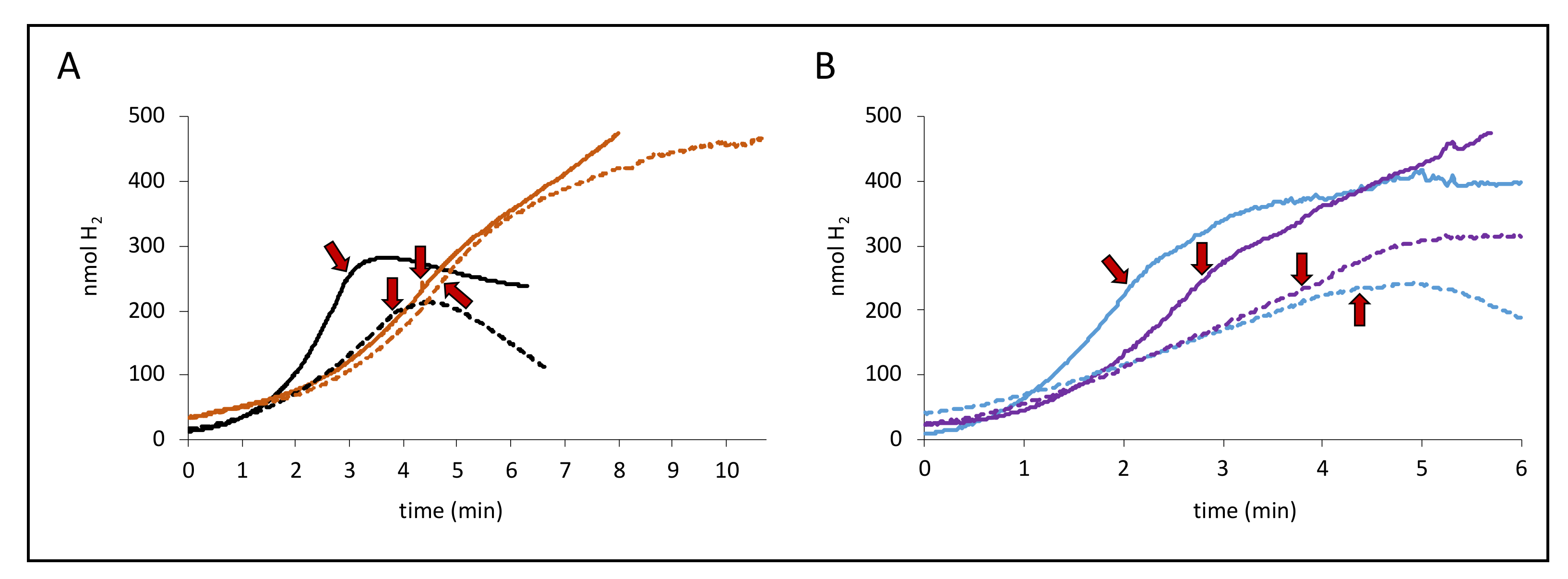
2.1. CCCP Inhibits H2 Production to Different Degrees Depending on the Hydrogenase Composition
2.2. Hyd-1 Confers Resistance and Hyd-2 Sensitivity of H2 Production to CCCP
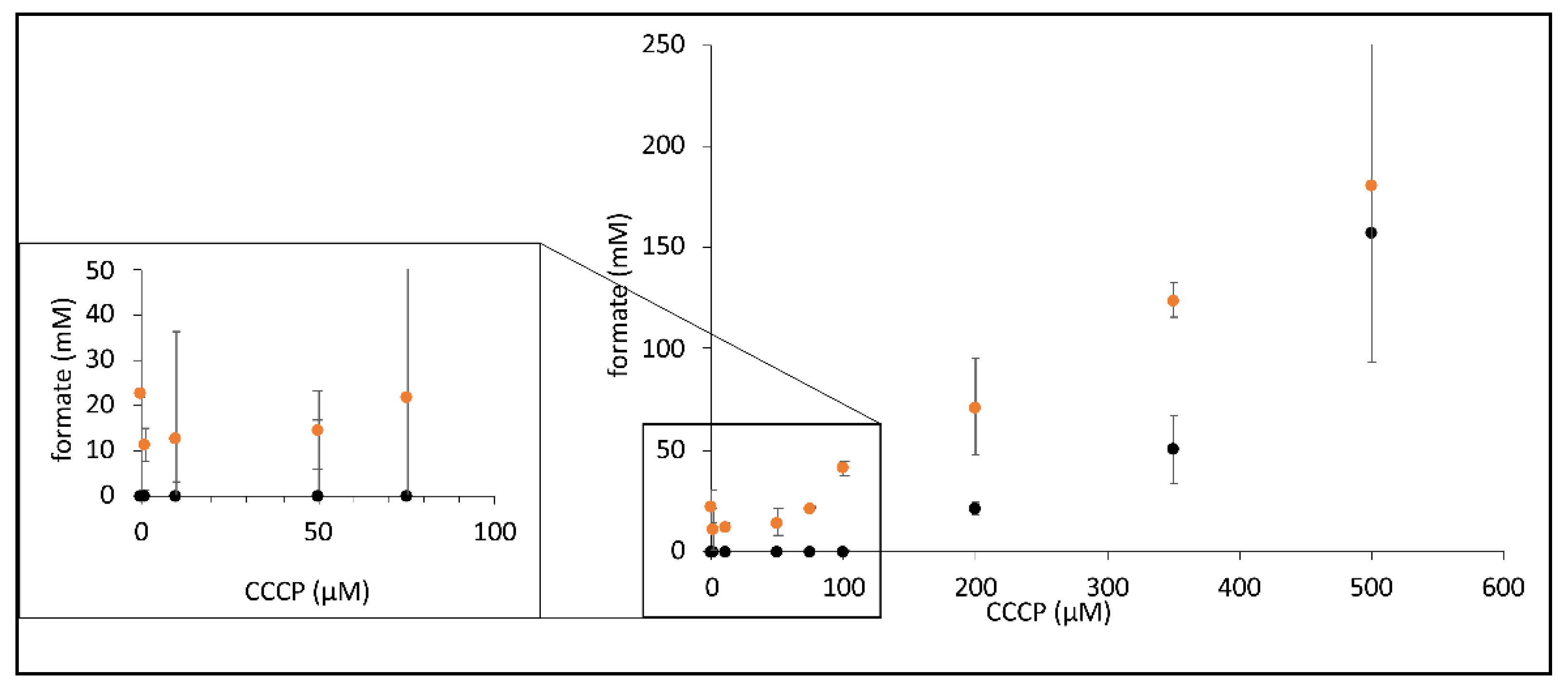
2.3. CCCP Promotes the Reverse FHL Reaction
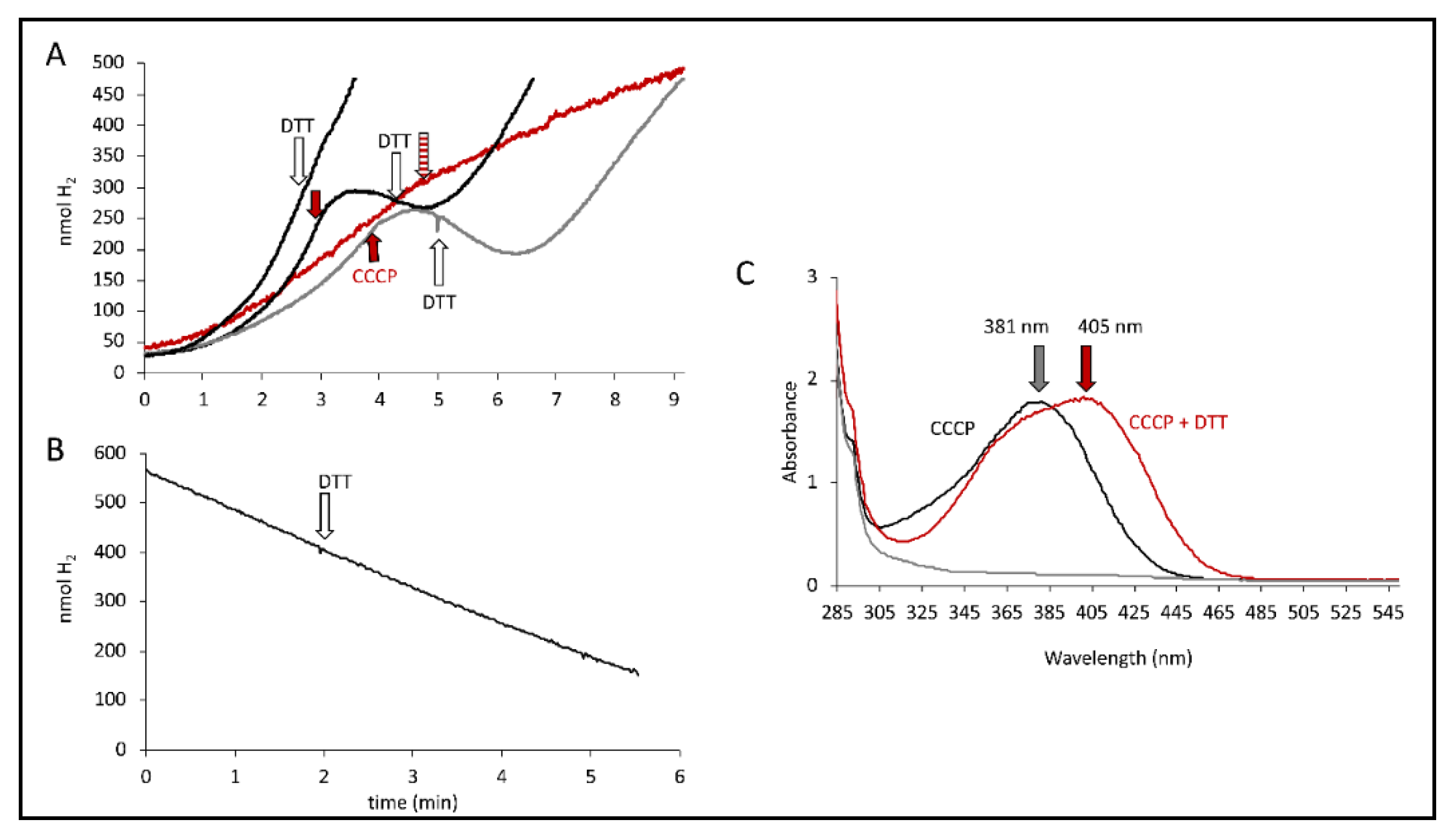
2.4. The DTT Reversal of the CCCP Effect Is Not Mediated by Hyd-2 Activity
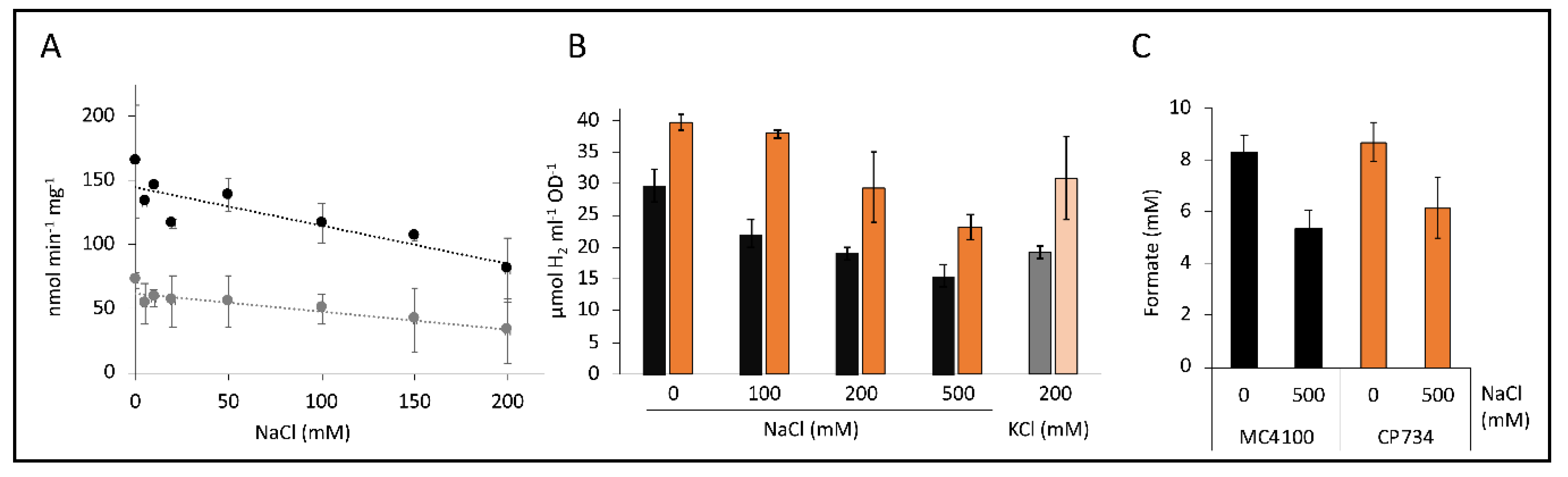
2.5. Effect of Monovalent Cations on FHL Reactions
2.6. The Na+ Ionophore EIPA Enhances CCCP Inhibition
3. Materials and Methods
3.1. Strains and Growth Conditions
3.2. Enzymatic Assays
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pinske, C. Bioenergetic aspects of archaeal and bacterial hydrogen metabolism. Adv. Microb. Physiol. 2019, 74, 487–514. [Google Scholar]
- Lubek, D.; Simon, A.H.; Pinske, C. Amino acid variants of the HybB membrane subunit of Escherichia coli [NiFe]-hydrogenase-2 support a role in proton transfer. FEBS Lett. 2019, 156, 2194–2203. [Google Scholar] [CrossRef]
- Pinske, C.; Sawers, R.G. Anaerobic formate and hydrogen metabolism. EcoSal Plus 2016, 7, ESP-0011-2016. [Google Scholar] [CrossRef]
- Odom, J.M.; Peck, H.D. Hydrogen cycling as a general mechanism for energy coupling in the sulfate-reducing bacteria, Desulfovibrio sp. FEMS Microbiol. Lett. 1981, 12, 47–50. [Google Scholar] [CrossRef]
- Wiechmann, A.; Ciurus, S.; Oswald, F.; Seiler, V.N.; Müller, V. It does not always take two to tango: “Syntrophy” via hydrogen cycling in one bacterial cell. ISME J. 2020, 14, 1561–1570. [Google Scholar] [CrossRef]
- Sawers, R.G. Formate and its role in hydrogen production in Escherichia coli. Biochem. Soc. Trans. 2005, 33, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Sauter, M.; Böhm, R.; Böck, A. Mutational analysis of the operon (hyc) determining hydrogenase 3 formation in Escherichia coli. Mol. Microbiol. 1992, 6, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Pinske, C.; Sargent, F. Exploring the directionality of Escherichia coli formate hydrogenlyase: A membrane-bound enzyme capable of fixing carbon dioxide to organic acid. Microbiologyopen 2016, 5, 721–737. [Google Scholar] [CrossRef] [PubMed]
- Roger, M.; Brown, F.; Gabrielli, W.; Sargent, F. Efficient hydrogen-dependent carbon dioxide reduction by Escherichia coli. Curr. Biol. 2018, 28, 140–145. [Google Scholar] [CrossRef] [Green Version]
- Redwood, M.D.; Mikheenko, I.P.; Sargent, F.; Macaskie, L.E. Dissecting the roles of Escherichia coli hydrogenases in biohydrogen production. FEMS Microbiol. Lett. 2008, 278, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Andrews, S.C.; Berks, B.C.; McClay, J.; Ambler, A.; Quail, M.A.; Golby, P.; Guest, J.R. A 12-cistron Escherichia coli operon (hyf) encoding a putative proton-translocating formate hydrogenlyase system. Microbiology 1997, 143, 3633–3647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böhm, R.; Sauter, M.; Böck, A. Nucleotide sequence and expression of an operon in Escherichia coli coding for formate hydrogenlyase components. Mol. Microbiol. 1990, 4, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Marreiros, B.C.; Batista, A.P.; Duarte, A.M.S.; Pereira, M.M. A missing link between complex I and group 4 membrane-bound [NiFe] hydrogenases. Biochim. Biophys. Acta 2012, 1827, 198–209. [Google Scholar] [CrossRef] [Green Version]
- Welte, C.; Krätzer, C.; Deppenmeier, U. Involvement of Ech hydrogenase in energy conservation of Methanosarcina mazei. FEBS J. 2010, 277, 3396–3403. [Google Scholar] [CrossRef] [PubMed]
- McDowall, J.S.; Murphy, B.J.; Haumann, M.; Palmer, T.; Armstrong, F.A.; Sargent, F. Bacterial formate hydrogenlyase complex. Proc. Natl. Acad. Sci. USA 2014, 111, E3948–E3956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokol, K.P.; Robinson, W.E.; Oliveira, A.R.; Zacarias, S.; Lee, C.-Y.; Madden, C.; Bassegoda, A.; Hirst, J.; Pereira, I.A.C.; Reisner, E. Reversible and selective interconversion of hydrogen and carbon dioxide into formate by a semiartificial formate hydrogenlyase mimic. J. Am. Chem. Soc. 2019, 141, 17498–17502. [Google Scholar] [CrossRef] [Green Version]
- Bagramyan, K.A.; Martirosov, S.M. Formation of an ion transport supercomplex in Escherichia coli. An experimental model of direct transduction of energy. FEBS Lett. 1989, 246, 149–152. [Google Scholar] [CrossRef] [Green Version]
- Babu, M.; Bundalovic-Torma, C.; Calmettes, C.; Phanse, S.; Zhang, Q.; Jiang, Y.; Minic, Z.; Kim, S.; Mehla, J.; Gagarinova, A.; et al. Global landscape of cell envelope protein complexes in Escherichia coli. Nat. Biotechnol. 2018, 36, 103–112. [Google Scholar] [CrossRef]
- Trchounian, A.; Sawers, R.G. Novel insights into the bioenergetics of mixed-acid fermentation: Can hydrogen and proton cycles combine to help maintain a proton motive force? IUBMB Life 2014, 66, 1–7. [Google Scholar] [CrossRef]
- Doberenz, C.; Zorn, M.; Falke, D.; Nannemann, D.; Hunger, D.; Beyer, L.; Ihling, C.H.; Meiler, J.; Sinz, A.; Sawers, R.G. Pyruvate formate-lyase interacts directly with the formate channel FocA to regulate formate translocation. J. Mol. Biol. 2014, 426, 2827–2839. [Google Scholar] [CrossRef] [Green Version]
- Beyer, L.; Doberenz, C.; Falke, D.; Hunger, D.; Suppmann, B.; Sawers, R.G. Coordination of FocA and pyruvate formate-lyase synthesis in Escherichia coli demonstrates preferential translocation of formate over other mixed-acid fermentation products. J. Bacteriol. 2013, 195, 1428–1435. [Google Scholar] [CrossRef] [Green Version]
- Hakobyan, M.; Sargsyan, H.; Bagramyan, K. Proton translocation coupled to formate oxidation in anaerobically grown fermenting Escherichia coli. Biophys. Chem. 2005, 115, 55–61. [Google Scholar] [CrossRef]
- Mayer, C.; Boos, W. Hexose/Pentose and Hexitol/Pentitol Metabolism. EcoSal Plus 2005, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Pinske, C.; Krüger, S.; Soboh, B.; Ihling, C.; Kuhns, M.; Braussemann, M.; Jaroschinsky, M.; Sauer, C.; Sargent, F.; Sinz, A.; et al. Efficient electron transfer from hydrogen to benzyl viologen by the [NiFe]-hydrogenases of Escherichia coli is dependent on the coexpression of the iron-sulfur cluster-containing small subunit. Arch. Microbiol. 2011, 193, 893–903. [Google Scholar] [CrossRef]
- Laurinavichene, T.V.; Tsygankov, A.A. H2 consumption by Escherichia coli coupled via hydrogenase 1 or hydrogenase 2 to different terminal electron acceptors. FEMS Microbiol. Lett. 2001, 202, 121–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawers, R.G.; Ballantine, S.; Boxer, D. Differential expression of hydrogenase isoenzymes in Escherichia coli K-12: Evidence for a third isoenzyme. J. Bacteriol. 1985, 164, 1324–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinske, C.; Jaroschinsky, M.; Linek, S.; Kelly, C.L.; Sargent, F.; Sawers, R.G. Physiology and bioenergetics of [NiFe]-hydrogenase 2-catalyzed H2-consuming and H2-producing reactions in Escherichia coli. J. Bacteriol. 2015, 197, 296–306. [Google Scholar] [CrossRef] [Green Version]
- Laurinavichene, T.V.; Zorin, N.A.; Tsygankov, A.A. Effect of redox potential on activity of hydrogenase 1 and hydrogenase 2 in Escherichia coli. Arch. Microbiol. 2002, 178, 437–442. [Google Scholar] [CrossRef]
- Kuniyoshi, T.M.; Balan, A.; Schenberg, A.C.G.; Severino, D.; Hallenbeck, P.C. Heterologous expression of proteorhodopsin enhances H2 production in Escherichia coli when endogenous Hyd-4 is overexpressed. J. Biotechnol. 2015, 206, 52–57. [Google Scholar] [CrossRef]
- Ridgway, H.F. Source of energy for gliding motility in Flexibacter polymorphus: Effects of metabolic and respiratory inhibitors on gliding movement. J. Bacteriol. 1977, 131, 544–556. [Google Scholar] [CrossRef] [Green Version]
- Šturdík, E.; Antalík, M.; Sulo, P.; Baláž, Š.; Ďurčová, E.; Drobnica, Ľ. Relationships among structure, reactivity towards thiols and basicity of phenylhydrazonopropanedinitriles. Collect. Czechoslov. Chem. Commun. 1985, 50, 2065–2076. [Google Scholar] [CrossRef]
- Gevorgyan, H.; Trchounian, A.; Trchounian, K. Formate and potassium ions affect Escherichia coli proton ATPase activity at low pH during mixed carbon fermentation. IUBMB Life 2020, 72, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y. Proton-motive force stimulates the proteolytic activity of FtsH, a membrane-bound ATP-dependent protease in Escherichia coli. Proc. Natl. Acad. Sci. USA 2002, 99, 8066–8071. [Google Scholar] [CrossRef] [Green Version]
- Rossmann, R.; Sawers, R.G.; Böck, A. Mechanism of regulation of the formate-hydrogenlyase pathway by oxygen, nitrate, and pH: Definition of the formate regulon. Mol. Microbiol. 1991, 5, 2807–2814. [Google Scholar] [CrossRef]
- Bagramyan, K.; Mnatsakanyan, N.; Poladian, A.; Vassilian, A.; Trchounian, A. The roles of hydrogenases 3 and 4, and the F0F1-ATPase, in H2 production by Escherichia coli at alkaline and acidic pH. FEBS Lett. 2002, 516, 172–178. [Google Scholar] [CrossRef] [Green Version]
- Kashket, E.R. Effects of K+ and Na+ on the proton motive force of respiring Escherichia coli at alkaline pH. J. Bacteriol. 1985, 163, 423–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verkhovskaya, M.L.; Verkhovsky, M.I.; Wikström, M. K+-dependent Na+ transport driven by respiration in Escherichia coli cells and membrane vesicles. Biochim. Biophys. Acta 1996, 1273, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Padan, E.; Landau, M. Sodium-proton (Na+/H+) antiporters: Properties and roles in health and disease. Met. Ions Life Sci. 2016, 16, 391–458. [Google Scholar]
- Friedrich, T.; Scheide, D. The respiratory complex I of bacteria, archaea and eukarya and its module common with membrane-bound multisubunit hydrogenases. FEBS Lett. 2000, 479, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Steuber, J. The C-terminally truncated NuoL subunit (ND5 homologue) of the Na+-dependent complex I from Escherichia coli transports Na+. J. Biol. Chem. 2003, 278, 26817–26822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steuber, J.; Schmid, C.; Rufibach, M.; Dimroth, P. Na+ translocation by complex I (NADH:quinone oxidoreductase) of Escherichia coli. Mol. Microbiol. 2000, 35, 428–434. [Google Scholar] [CrossRef]
- Stolpe, S.; Friedrich, T. The Escherichia coli NADH:ubiquinone oxidoreductase (complex I) is a primary proton pump but may be capable of secondary sodium antiport. J. Biol. Chem. 2004, 279, 18377–18383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.J.; Lee, H.S.; Kim, E.S.; Bae, S.S.; Lim, J.K.; Matsumi, R.; Lebedinsky, A.V.; Sokolova, T.G.; Kozhevnikova, D.A.; Cha, S.-S.; et al. Formate-driven growth coupled with H2 production. Nature 2010, 467, 352–355. [Google Scholar] [CrossRef]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.; Tomita, M.; Wanner, B.; Mori, H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006, 2, 2006.0008. [Google Scholar] [CrossRef] [Green Version]
- Begg, Y.A.; Whyte, J.N.; Haddock, B.A. The identification of mutants of Escherichia coli deficient in formate dehydrogenase and nitrate reductase activities using dye indicator plates. FEMS Microbiol. Lett. 1977, 2, 47–50. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Casadaban, M.J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J. Mol. Biol. 1976, 104, 541–555. [Google Scholar] [CrossRef]
- Jacobi, A.; Rossmann, R.; Böck, A. The hyp operon gene products are required for the maturation of catalytically active hydrogenase isoenzymes in Escherichia coli. Arch. Microbiol. 1992, 158, 444–451. [Google Scholar] [CrossRef]
- Sargent, F.; Stanley, N.R.; Berks, B.C.; Palmer, T. Sec-independent protein translocation in Escherichia coli. A distinct and pivotal role for the TatB protein. J. Biol. Chem. 1999, 274, 36073–36082. [Google Scholar] [CrossRef] [Green Version]
- Lowry, O.; Rosebrough, N.; Farr, A.; Randall, R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]



| Strain 1 | Glucose | Formate |
|---|---|---|
| MC4100 | 69 ± 12 | 160 ± 47 |
| CP630 (ΔhyaB) | 74 ± 23 | 173 ± 13 |
| CP631 (ΔhybC) | 101 ± 15 | 145 ± 20 |
| CP734 (ΔhyaB ΔhybC) | 114 ± 24 | 121 ± 13 |
| Strain 1 | Glucose | Formate |
|---|---|---|
| MC4100 | 31 µM | 81 µM |
| CP630 (ΔhyaB) | 0.13 µM | 42 µM |
| CP631(ΔhybC) | 125 µM | 223 µM |
| CP734 (ΔhyaB ΔhybC) | 98 µM | 348 µM |
| Strain | Genotype | Reference |
|---|---|---|
| MC4100 | F- araD139 Δ(argF-lac)U169 λ rpsL150 relA1 deoC1 flhD5301 Δ(fruK-yeiR)725(fruA25), rbsR22, Δ(fimB-fimE)632(::IS1) | [47] |
| CP630 | Like MC4100, but ΔhyaB | This study |
| CP631 | Like MC4100, but ΔhybC | This study |
| CP734 | Like MC4100, but ΔhyaB ΔhybC | [24] |
| HDK103 | Like MC4100, but ΔhyaB ΔhycA-I | [48] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Telleria Marloth, J.; Pinske, C. Susceptibility of the Formate Hydrogenlyase Reaction to the Protonophore CCCP Depends on the Total Hydrogenase Composition. Inorganics 2020, 8, 38. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics8060038
Telleria Marloth J, Pinske C. Susceptibility of the Formate Hydrogenlyase Reaction to the Protonophore CCCP Depends on the Total Hydrogenase Composition. Inorganics. 2020; 8(6):38. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics8060038
Chicago/Turabian StyleTelleria Marloth, Janik, and Constanze Pinske. 2020. "Susceptibility of the Formate Hydrogenlyase Reaction to the Protonophore CCCP Depends on the Total Hydrogenase Composition" Inorganics 8, no. 6: 38. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics8060038