Chiral or Luminescent Lanthanide Single-Molecule Magnets Involving Bridging Redox Active Triad Ligand
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystal Structure Description of [Yb2(hfac)6(L)]·2(C7H16) ((1)·2(C7H16))
2.2. Crystal Structure Description of [Dy2((+)facam)6(L)]·2(C6H14) ((2)·2(C6H14))
2.3. Electrochemical Properties
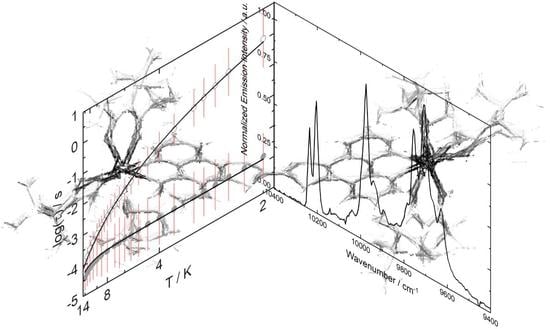
2.4. Photophysical Properties
2.5. Magnetic Properties
3. Experimental Section
3.1. Synthesis of [Yb2(hfac)6(L)]·2(C7H16) ((1)·2(C7H16))
3.2. Synthesis of Complex {[Dy(hfac)3(L)]·2C6H14}n 1
3.3. Synthesis of [Dy2((+)facam)6(L)]·2(C6H14) ((2)·2(C6H14))
3.4. Crystallography
3.5. Physical Measurements
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SMM | Single-Molecule Magnet |
| QTM | Quantum Tunneling of the Magnetization |
| Hfac− | 1,1,1,5,5,5-hexafluoroacetylacetonato anion |
| Facam− | 3-trifluoro-acetyl-(+)-camphorato anion |
| Phen | 1,10-phenantroline |
| TTF | Tetrathiafulvalene |
| HOMO | Highest Occupied Molecular Orbital |
| LUMO | Lowest Unoccupied Molecular Orbital |
| ILCT | Intra-Ligand Charge Transfer |
| CH2Cl2 | dichloromethane |
References
- Sessoli, R.; Powell, A.K. Strategies towards single molecule magnets based on lanthanide ions. Coord. Chem. Rev. 2009, 253, 2328–2341. [Google Scholar] [CrossRef]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide single-molecule magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Guo, Y.-N.; Tang, J. Recent advances in dysprosium-based single molecule magnets: Structural overview and synthetic strategies. Coord. Chem. Rev. 2013, 257, 1728–1763. [Google Scholar] [CrossRef]
- Ungur, L.; Lin, S.-Y.; Tang, J.; Chibotaru, L.F. Single-molecule toroics in Ising-type lanthanide molecular clusters. Chem. Soc. Rev. 2014, 43, 6894–6905. [Google Scholar] [CrossRef] [PubMed]
- Feltham, H.L.C.; Brooker, S. Review of purely 4f and mixed-metal nd-4f single-molecule magnets containing only one lanthanide ion. Coord. Chem. Rev. 2014, 276, 1–33. [Google Scholar] [CrossRef]
- Liddle, S.T.; Van Slageren, J. Improving f-element single molecule magnets. Chem. Soc. Rev. 2015, 44, 6655–6669. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.K.; Murugavel, R. Enriching lanthanide single-ion magnetism through symmetry and axiality. Chem. Commun. 2018, 54, 3685–3696. [Google Scholar] [CrossRef]
- Liu, J.-L.; Chen, Y.-C.; Tong, M.-L. Symmetry strategies for high performance lanthanide-based single-molecule magnets. Chem. Soc. Rev. 2018, 47, 2431–2453. [Google Scholar] [CrossRef]
- Zhu, Z.; Guo, M.; Li, X.-L.; Tang, J. Molecular magnetism of lanthanide: Advances and perspectives. Coord. Chem. Rev. 2019, 378, 350–364. [Google Scholar] [CrossRef]
- Parmar, V.S.; Mills, D.P.; Winpenny, R.E.P. Mononuclear Dysprosium Alkoxide and Aryloxide Single-Molecule Magnets. Chem. Eur. J. 2021, 27, 7625–7645. [Google Scholar] [CrossRef]
- Goodwin, C.A.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular magnetic hysteresis at 60 kelvin in dysprocenium. Nature 2017, 548, 439–442. [Google Scholar] [CrossRef]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef] [Green Version]
- Aromi, G.; Aguila, D.; Gamez, P.; Luis, F.; Roubeau, O. Design of magnetic coordination complexes for quantum computing. Chem. Soc. Rev. 2012, 41, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Thiele, S.; Balestro, F.; Ballou, R.; Klyatskaya, S.; Ruben, M.; Wernsdorfer, W. Electrically driven nuclear spin reconance in single-molecule magnets. Science 2014, 344, 1135–1138. [Google Scholar] [CrossRef]
- Piguet, C.; Bünzli, J.-C.G. Mono-and polymetallic lanthanide-containing functional assemblies: A field between tradition and novelty. Chem. Soc. Rev. 1999, 28, 347–358. [Google Scholar] [CrossRef]
- Comby, S.; Bünzli, J.-C.G. Handbook on the Physics and Chemistry of Rare Earths; Elsevier BV: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Cucinotta, G.; Perfetti, M.; Luzon, J.; Etienne, M.; Car, P.-E.; Caneschi, A.; Calvez, G.; Bernot, K.; Sessoli, R. Magnetic Anisotropy in a Dysprosium/DOTA Single-Molecule Magnet: Beyond Simple Magneto-Structural Correlations. Angew. Chem. Int. Ed. 2012, 51, 1606–1610. [Google Scholar] [CrossRef]
- Pointillart, F.; Le Guennic, B.; Cador, O.; Maury, O.; Ouahab, L. Lanthanide Ion and Tetrathiafulvalene-Based Ligand as a “magic” Couple toward Luminescence, Single-Molecule Magnets, and Magnetostructural Correlations. Acc. Chem. Res. 2015, 48, 2834–2842. [Google Scholar] [CrossRef]
- Jia, J.-H.; Li, Q.-W.; Chen, Y.-C.; Liu, J.-L.; Tong, M.-L. Luminescent single-molecule magnets based on lanthanides: Design strategies, recent advances and magneto-luminescent studies. Coord. Chem. Rev. 2019, 378, 365–381. [Google Scholar] [CrossRef]
- Gendron, F.; Di Pietro, S.; Galan, L.A.; Riobé, F.; Placide, V.; Guy, L.; Zinna, F.; Di Bari, L.; Bensalah-Ledoux, A.; Guyot, Y.; et al. Luminescence, chiroptical, magnetic and ab initio crystal-field characterizations of an enantiopure helicoidal Yb(III) complex. Inorg. Chem. Front. 2021, 8, 914–926. [Google Scholar] [CrossRef]
- Li, X.-J.; Chen, C.-L.; Xiao, H.-P.; Wang, A.-L.; Liu, C.-M.; Zheng, X.; Gao, L.-J.; Yanga, X.-G.; Fang, S.-M. Luminescent, magnetic and ferroelectric properties of noncentrosymmetric chain-like complexes composed of ninecoordinate lanthanide ions. Dalton Trans. 2013, 42, 15317–15325. [Google Scholar] [CrossRef]
- Long, J.; Vallat, R.; Ferreira, R.A.S.; Carlos, L.D.; Almeida Paz, F.A.; Guari, Y.; Larionova, J. A bifunctional luminescent single-ion magnet: Towards correlation between luminescence studies and magnetic slow relaxation processes. Chem. Commun. 2012, 48, 9974–9976. [Google Scholar] [CrossRef] [PubMed]
- Pointillart, F.; Le Guennic, B.; Cauchy, T.; Golhen, S.; Cador, O.; Maury, O.; Ouahab, L. A Series of Tetrathiafulvalene-Based Lanthanide Complexes Displaying Either Single Molecule Magnet or Luminescence-Direct Magnetic and Photo-Physical Correlations in the Ytterbium Analogue. Inorg. Chem. 2013, 52, 5978–5990. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Bao, S.; Wang, B.-W.; Ferreira, R.A.S.; Zheng, L.-M.; Carlos, L.D. Lanthanide phosphonates with pseudo-D5 h local symmetry exhibiting magnetic and luminescence bifunctional properties. Inorg. Chem. Front. 2015, 2, 558–566. [Google Scholar] [CrossRef]
- Long, J.; Mamontova, E.; Freitas, V.; Luneau, D.; Vieru, V.; Chibotaru, L.F.; Ferreira, R.A.S.; Félix, G.; Guari, Y.; Carlos, L.D.; et al. Study of the influence of magnetic dilution over relaxation processes in a Zn/Dy single-ion magnet by correlation between luminescence and magnetism. RSC Adv. 2016, 6, 108810–108818. [Google Scholar] [CrossRef]
- Brunet, G.; Marin, R.; Monk, M.; Resch-Genger, U.; Galico, D.A.; Sigoli, F.A.; Suturina, E.A.; Hemmer, E.; Murugesu, M. Exploring the dual functionality of an ytterbium complex for luminescence thermometry and slow magnetic relaxation. Chem. Sci. 2019, 10, 6799–6808. [Google Scholar] [CrossRef]
- Marin, R.; Brunet, G.; Murugesu, M. Shining New Light on Multifunctional Lanthanide Single-Molecule Magnets. Angew. Chem. Int. Ed. 2021, 60, 1728–1746. [Google Scholar] [CrossRef]
- Guettas, D.; Gendron, F.; Fernandez Garcia, G.; Riobé, F.; Roisnel, T.; Maury, O.; Pilet, G.; Cador, O.; Le Guennic, B. Luminescence-Driven Electronic Structure Determination in a Textbook Dimeric Dy(III)-based Single Molecule Magnet. Chem. Eur. J. 2020, 26, 4389–4395. [Google Scholar] [CrossRef]
- Casanovas, B.; Speed, S.; El Fallah, M.S.; Vicente, R.; Font-Bardia, M.; Zinna, F.; Di Bari, L. Chiral dinuclear Ln(III) complexes derived from S-and R-2-(6-methoxy-2-naphthyl) propionate. Optical and magnetic properties. Dalton Trans. 2019, 48, 2059–2067. [Google Scholar] [CrossRef]
- Li, D.-P.; Wang, T.-W.; Li, C.-H.; Liu, D.-S.; Li, Y.-Z.; You, X.-Z. Single-ion magnets based on mononuclear lanthanide complexes with chiral Schiff base ligands [Ln(FTA)3L] (Ln = Sm, Eu, Gd, Tb and Dy). Chem. Commun. 2010, 46, 2929–2931. [Google Scholar] [CrossRef]
- Li, X.-L.; Chen, C.-L.; Gao, Y.-L.; Liu, C.-M.; Feng, X.-L.; Gui, Y.-H.; Fang, S.-M. Modulation of Homochiral DyIII Complexes: Single-Molecule Magnets with Ferroelectric Properties. Chem. Eur. J. 2012, 18, 14632–14637. [Google Scholar] [CrossRef]
- Long, J.; Ivanov, M.; Khomchenko, V.; Mamontova, E.; Thibaud, J.-M.; Rouquette, J.; Beaudhuin, M.; Granier, D.; Ferreira, R.A.S.; Carlos, L.; et al. Room temperature magnetoelectric coupling in a molecular ferroelectric ytterbium(III) complex. Science 2020, 367, 671–676. [Google Scholar] [CrossRef]
- Long, J.; Rouquette, J.; Thibaud, J.-M.; Ferreira, R.A.R.; Carlos, L.D.; Donnadieu, B.; Vieru, V.; Chibotaru, L.F.; Konczewicz, L.; Haines, J.; et al. A High—Temperature Molecular Ferroelectric Zn/Dy Complex Exhibiting Single-Ion-Magnet Behavior and Lanthanide Luminescence. Angew. Chem. Int. Ed. 2015, 54, 2236–2240. [Google Scholar] [CrossRef]
- Li, X.-L.; Hu, M.; Yin, Z.; Zhu, C.; Liu, C.-M.; Xiao, H.-P.; Fang, S. Enhancement single-ion magnetic and ferroelectric properties of mononuclear Dy(III) enantiomeric pairs through the coordination role of chiral ligands. Chem. Commun. 2017, 53, 3998–4001. [Google Scholar] [CrossRef]
- Mattei, C.A.; Montigaud, V.; Gendron, F.; Denis-Quanquin, S.; Dorcet, V.; Giraud, N.; Riobé, F.; Argouarch, G.; Maury, O.; Le Guennic, B.; et al. Solid-state versus solution investigation of a luminescent chiral BINOL-derived bisphosphate single-molecule magnet. Inorg. Chem. Front. 2021, 8, 947–962. [Google Scholar] [CrossRef]
- Mattei, C.A.; Montigaud, V.; Dorcet, V.; Riobé, F.; Argouarch, G.; Maury, O.; Le Guennic, B.; Cador, O.; Lalli, C.; Pointillart, F. Luminescent dysprosium single-molecule magnets made from designed chiral BINOL-derived bisphosphate ligands. Inorg. Chem. Front. 2021, 8, 963–976. [Google Scholar] [CrossRef]
- Wang, K.; Zeng, S.; Wang, H.; Dou, J.; Jiang, J. Magnetochiral dichroism in chiral mixed (phthalocyaninato)(porphyrinato) rare earth triple-decker SMMs. Inorg. Chem. Front. 2014, 1, 167–171. [Google Scholar] [CrossRef]
- Atzori, M.; Dhbaibi, K.; Douib, H.; Grasser, M.; Dorcet, V.; Breslavetz, I.; Paillot, K.; Cador, O.; Rikken, G.L.J.A.; Le Guennic, B.; et al. Helicene-Based Ligands Enable Strong Magneto-Chiral Dichroism in a Chiral Ytterbium Complex. J. Am. Chem. Soc. 2021, 143, 2671–2675. [Google Scholar] [CrossRef]
- Zhang, P.; Perfetti, M.; Kern, M.; Hallmen, P.P.; Ungur, L.; Lenz, S.; Ringenberg, M.R.; Frey, W.; Stoll, H.; Rauhut, G.; et al. Exchange coupling and single molecule magnetism in redox-active tetraoxolene-bridged dilanthanide complexes. Chem. Sci. 2018, 9, 1221–1230. [Google Scholar] [CrossRef] [Green Version]
- Dolinar, B.S.; Gomez-Coca, S.; Alexandropoulos, D.I.; Dunbar, K.R. An air stable radical-bridged dysprosium single molecule magnet and its neutral counterpart: Redox switching of magnetic relaxation dynamics. Chem. Commun. 2017, 53, 2283–2286. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Zhukov, P.A.; Tomilova, L.G.; Zefirov, N.S. New diphthalocyanine complexes of rare-earth metals based on 4,5-isopropylidenedioxyphthalonitrile. Russ. Chem. Bull. 2006, 55, 281–286. [Google Scholar] [CrossRef]
- Takamatsu, S.; Isikawa, T.; Koshihara, S.-Y.; Ishikawa, N. Significant increase of the barrier energy for magnetization reversal of a single-4f-ionic single-molecule magnet by a longitudinal contraction of the coordination space. Inorg. Chem. 2007, 46, 7250–7252. [Google Scholar] [CrossRef] [PubMed]
- Cador, O.; Le Guennic, B.; Pointillart, F. Electro-activity and magnetic switching in lanthanide-based single-molecule magnets. Inorg. Chem. Front. 2019, 6, 3398–3417. [Google Scholar] [CrossRef] [Green Version]
- Lefeuvre, B.; Flores Gonzalez, J.; Gendron, F.; Dorcet, V.; Riobé, F.; Cherkasov, V.; Maury, O.; Le Guennic, B.; Cador, O.; Kuropatov, V.; et al. Redox-Modulations of Photophysical and Single-Molecule Magnet Properties in Ytterbium Complexes Involving Extended-TTF Triads. Molecules 2020, 25, 492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pointillart, F.; Flores Gonzalez, J.; Montigaud, V.; Tesi, L.; Cherkasov, V.; Le Guennic, B.; Cador, O.; Ouahab, L.; Sessoli, R.; Kuropatov, V. Redox- and solvato-magnetic switching in a tetrathiafulvalene-based triad single-molecule magnet. Inorg. Chem. Front. 2020, 7, 2322–2334. [Google Scholar] [CrossRef]
- Tiaouinine, S.; Flores Gonzalez, J.; Montigaud, V.; Mattei, C.A.; Dorcet, V.; Kaboub, L.; Cherkasov, V.; Cador, O.; Le Guennic, B.; Ouahab, L.; et al. Redox Modulation of Field-Induced Tetrathiafulvalene-Based Single-Molecule Magnets of Dysprosium. Magnetochemistry 2020, 6, 34. [Google Scholar] [CrossRef]
- Lorcy, D.; Bellec, N.; Fourmigué, M.; Avarvari, N. tetrathiafulvalene-based group XV ligands: Synthesis, coordination chemistry and radical cation salts. Coord. Chem. Rev. 2009, 253, 1398–1438. [Google Scholar] [CrossRef]
- Pointillart, F.; Golhen, S.; Cador, O.; Ouahab, L. 3d and 4d coordination complexes and coordination polymers involving electroactive tetrathiafulvalene containing ligands. C. R. Chimie 2013, 16, 679–687. [Google Scholar] [CrossRef] [Green Version]
- Pointillart, F.; Golhen, S.; Cador, O.; Ouahab, L. Paramagnetic 3d coordination complexes involving redox-active tetrathiafulvalene derivatives: An efficient approach to elaborate multi-properties materials. Dalton Trans. 2013, 42, 1949–1960. [Google Scholar] [CrossRef]
- Kuropatov, V.; Klementieva, S.; Fukin, G.; Mitin, A.; Ketkov, S.; Budnikova, Y.; Cherkasov, V.; Abakumov, G. Novel method for the synthesis of functionalized tetrathiafulvalenes, an acceptor–donor–acceptor molecule comprising of two o-quinone moieties linked by a TTF bridge. Tetrahedron 2010, 66, 7605–7611. [Google Scholar] [CrossRef]
- Pointillart, F.; Klementieva, S.; Kuropatov, V.; Le Gal, Y.; Golhen, S.; Cador, O.; Cherkasov, V.; Ouahab, L. A single molecule magnet behaviour in a D3h symmetry Dy(III) complex involving a quinone–tetrathiafulvalene–quinone bridge. Chem. Commun. 2012, 48, 714–716. [Google Scholar] [CrossRef]
- Jia, C.; Liu, S.X.; Tanner, C.; Leiggener, C.; Neels, A.; Sanguinet, L.; Levillain, E.; Leutwyler, S.; Hauser, A.; Decurtins, S. An experimental and computational study on intramolecular charge transfer: A tetrathiafulvalene-fused dipyridophenazine molecule. Chem. Eur. J. 2007, 13, 3804–3812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keniley, L., Jr.; Ray, L.; Kovnir, K.A.; Shatruk, M. TTF-annulated phenanthroline and unexpected oxidation cleavage of the C=C bond in its ruthenium(II) complex. Inorg. Chem. 2010, 49, 1307–1309. [Google Scholar] [CrossRef] [PubMed]
- Keniley, L., Jr.; Dupont, N.; Ray, L.; Ding, J.; Kovnir, K.; Hoyt, M.J.; Hauser, A.; Shatruk, M. Complexes with redox-active ligands: Synthesis, structure, and electrochemical and photophysical behavior of the Ru(II) complex with TTF-annulated phenanthroline. Inorg. Chem. 2013, 52, 8040–8052. [Google Scholar] [CrossRef]
- Qin, J.; Hu, L.; Li, G.N.; Wang, X.S.; Xu, Y.; Zuo, J.-L.; You, X.-Z. Synthesis, characterization, and properties of rhenium(I). tricarbonyl complexes with tetrathiafulvalene-fused phenanthroline ligands. Organometallics 2011, 30, 2173–2179. [Google Scholar] [CrossRef]
- Jia, H.; Ding, J.; Hauser, A.; Decurtins, S.; Liu, S.-X. Large p-conjugated chromophores derived from tetrathiafulvalene. Asian J. Org. Chem. 2014, 3, 198–202. [Google Scholar] [CrossRef]
- Chen, B.; Lv, Z.-P.; Hua, C.F.; Leong, C.; Tuna, F.M.; D’Alessandro, D.; Collison, D.; Zuo, J.-L. Dinuclear ruthenium complex based on a p-extended bridging ligand with redox-active tetrathiafulvalene and 1,10-phenanthroline units. Inorg. Chem. 2016, 55, 4606–4615. [Google Scholar] [CrossRef]
- Lefeuvre, B.; Mattei, C.A.; Flores Gonzalez, J.; Gendron, F.; Dorcet, V.; Riobé, F.; Lalli, C.; Le Guennic, B.; Cador, O.; Maury, O.; et al. Solid-State Near-Infrared Circularly Polarized Luminescence from Chiral YbIII-Single-Molecule Magnet. Chem. Eur. J. 2021, 27, 7362–7366. [Google Scholar] [CrossRef]
- Lefeuvre, B.; Galangau, O.; Flores Gonzalez, J.; Montigaud, V.; Dorcet, V.; Ouahab, L.; Le Guennic, B.; Cador, O.; Pointillart, F. Field-Induced Dysprosium Single-Molecule Magnet Based on a Redox-Active Fused 1,10-Phenantroline-Tetrathiafulavlene-1,10-Phannatroline Bridging Triad. Front. Chem. 2018, 6, 552–561. [Google Scholar] [CrossRef] [Green Version]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE Program for the Stereochemical Analysis of Molecular Fragments by Means of Continuous Shape Measures and Associated Tools; Departament de Quimica Fisica, Departament de Quimica Inorganica, and Institut de Quimica Teorica i Computacional—Universitat dè Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Jones, A.E.; Christensen, C.A.; Perepichka, D.F.; Batsanov, A.S.; Beeby, A.; Low, P.J.; Bryce, M.R.; Parker, A.W. Photochemistry of the pi-extended 9,10-bis (1,3-dithiol-2-ylidene)-9,10-dihydroanthracene system: Generation and characterization of the radical cation, dication, and derived products. Chem. Eur. J. 2001, 7, 973–978. [Google Scholar] [CrossRef]
- Ellern, A.; Bernstein, J.; Becker, J.Y.; Zamir, S.; Shahal, L.; Cohen, S. A New Polymorphic Modification of Tetrathiafulvalene. Crystal Structure, Lattice Energy and Intermolecular Interactions. Chem. Mater. 1994, 6, 1378–1385. [Google Scholar] [CrossRef]
- Bünzli, J.C.G. Benefiting from the Unique properties of Lanthanide Ions. Acc. Chem. Res. 2006, 39, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.D.; Long, J.R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Goldner, P.; Pellé, F.; Meichenin, D.; Auzel, F. Cooperative luminescence in ytterbium-doped CsCdBr3. J. Lumin. 1997, 71, 137–150. [Google Scholar] [CrossRef]
- Yi, X.; Bernot, K.; Le Corre, V.; Calvez, G.; Pointillart, F.; Cador, O.; Le Guennic, B.; Jung, J.; Maury, O.; Placide, V.; et al. Unraveling the Crystal Structure of Lanthanide-Murexide Complexes: Use of an Ancient Complexometry Indicator as a Near-Infrared-Emitting Single-Ion Magnet. Chem. Eur. J. 2014, 20, 1569–1576. [Google Scholar] [CrossRef]
- Shavaleev, N.M.; Scopelliti, R.; Gumy, F.; Bünzli, J.-C.G. Surprisingly Bright Near-Infrared Luminescence and Short Radiative Lifetimes of Ytterbium in Hetero-Binuclear Yb-Na Chelates. Inorg. Chem. 2009, 48, 7937–7946. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Dreiser, J.; Weihe, H.; Sibille, R.; Johannesen, H.V.; Sorensen, M.A.; Nielsen, B.E.; Sigrist, M.; Mutka, H.; Rols, S.; et al. Design of Single-Molecule Magnets: Insufficiency of the Anisotropy Barrier as the Sole Criterion. Inorg. Chem. 2015, 54, 7600–7606. [Google Scholar] [CrossRef]
- Guégan, F.; Jung, J.; Le Guennic, B.; Riobé, F.; Maury, O.; Gillon, B.; Jasquot, Y.; Guyot, Y.; Morell, C.; Luneau, D. Evidencing under-barrier phenomena in a Yb(III) SMM: A joint luminescence/neutron diffraction/SQUID study. Inorg. Chem. Front. 2019, 6, 3152–3157. [Google Scholar] [CrossRef]
- Marbey, J.; Kragskow, J.G.; Buch, C.D.; Nehrkorn, J.; Ozerov, M.; Piligkos, S.; Hill, S.; Chilton, N. Vibronic Coupling in a Molecular 4f Qubit. ChemRxiv Prepr. 2021. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH: Weinhem, Germany, 1993. [Google Scholar]
- Car, P.-E.; Perfetti, M.; Mannini, M.; Favre, A.; Caneschi, A.; Sessoli, R. Giant field dependence of the low temperature relaxation of the magnetization in a dysprosium(iii)–DOTA complex. Chem. Commun. 2011, 47, 3751–3753. [Google Scholar] [CrossRef]
- Reta, D.; Chilton, N.F. Uncertainty estimates for magnetic relaxation times and magnetic relaxation parameters. Phys. Chem. Chem. Phys. 2019, 21, 23567–23575. [Google Scholar] [CrossRef]
- Abragam, A.; Bleaney, B. Electron Paramagnetic Resonance of Transition Ions; Clarendon Press: Oxford, UK, 1970. [Google Scholar]
- Singh, A.; Shrivastava, K.N. Optical-acoustic two-phonon relaxation in spin systems. Phys. Status Solidi B 1979, 95, 273. [Google Scholar] [CrossRef]
- Shirivastava, K.N. Theory of Spin-Lattice Relaxation. Phys. Status Solidi B 1983, 177, 437. [Google Scholar] [CrossRef]
- Evans, P.; Reta, D.; Whitehead, G.F.S.; Chilton, N.F.; Mills, D.P. Bis-Monophospholyl Dysprosium Cation Showing Magnetic Hysteresis at 48 K. J. Am. Chem. Soc. 2019, 141, 19935–19940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Ortega, I.F.; Herrera, J.M.; Carmona, A.R.; Galan-Mascaros, J.R.; Dey, S.; Nojiri, H.; Rajaraman, G.; Colacio, E. A Chiral Bipyrimidine-Bridged Dy2 SMM: A Comparative Experimental and Theoretical Study of the Correlation Between the Distortion of the DyO6N2 Coordination Sphere and the Anisotropy Barrier. Front. Chem. 2018, 6, 537–554. [Google Scholar] [CrossRef]
- Richardson, M.F.; Wagner, W.F.; Sands, D.E. Rare-earth trishexafluoroacetylacetonates and related compounds. J. Inorg. Nucl. Chem. 1968, 30, 1275–1289. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lefeuvre, B.; Flores Gonzalez, J.; Mattei, C.A.; Dorcet, V.; Cador, O.; Pointillart, F. Chiral or Luminescent Lanthanide Single-Molecule Magnets Involving Bridging Redox Active Triad Ligand. Inorganics 2021, 9, 50. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics9070050
Lefeuvre B, Flores Gonzalez J, Mattei CA, Dorcet V, Cador O, Pointillart F. Chiral or Luminescent Lanthanide Single-Molecule Magnets Involving Bridging Redox Active Triad Ligand. Inorganics. 2021; 9(7):50. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics9070050
Chicago/Turabian StyleLefeuvre, Bertrand, Jessica Flores Gonzalez, Carlo Andrea Mattei, Vincent Dorcet, Olivier Cador, and Fabrice Pointillart. 2021. "Chiral or Luminescent Lanthanide Single-Molecule Magnets Involving Bridging Redox Active Triad Ligand" Inorganics 9, no. 7: 50. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics9070050