1. Introduction
Polyoxidometalates (POMs) are defined as clusters made from early transition-metals, typically d
0 species V(V), Nb(V), Ta(V), Mo(VI), and W(VI), bridged by oxide anions. This class of compounds is highly interesting in molecular structural variety, reactivity, and applications in analytical chemistry, catalysis, medicine, and materials research [
1]. POMs have great potential in biological applications, since every aspect that involves the interaction of POM with biological target macromolecules could be modified to improve their beneficial effects on a biological system. Thus, interesting POMs with anticancer [
2] and antibiotic activities [
3], among others, have been obtained to date.
In this context, the colorless aqueous solution of vanadate(V) turns orange as it acidifies. This phenomenon is associated with condensation reactions carried out by vanadate ions depending on the acidity range of the solution. Thus, at pH ≈ 6, the orange solution indicates that the decavanadate anion, [H
nV
10O
28]
(6−n)− with
n = 0–4, has been formed [
4,
5]. These clusters have attracted much interest due to their potential applications in a wide range of uses such as sensors [
6], batteries [
7,
8,
9], catalysts [
10,
11,
12], or metallodrugs [
13,
14,
15].
In particular, decavanadates have recently attracted attention due to their medicinal and biochemical behavior, since they have an important role in biological systems by having the ability to interact with proteins, enzymes, and cell membranes [
16].
More than forty years ago, vanadate was first found as an impurity in commercial ATP obtained from horse skeletal muscle and was initially identified as a muscle inhibition factor by inhibiting the activity of the sodium pump [
17]. Subsequently, the first enzyme that decavanadate was able to inhibit, the rabbit skeletal muscle adenylate kinase, was reported. After that, many enzymes have been found that can be inhibited by this decameric species, such as hexokinase, phosphofructokinase, inositol phosphate metabolism enzymes, or nicotinamide adenine dinucleotide (NADH)-vanadate reductase [
18].
Due to the high negative charge of decavanadate, this species can interact with a multitude of molecules such as proteins, counterions, or lipid structures, affecting many biological processes such as muscle contraction, calcium homeostasis, necrosis, actin polymerization, oxidative stress markers, or glucose uptake, among others [
19]. As a result, different compounds based on decavanadate and cationic organic ligands have been published in recent years, which can decrease glycemia [
20,
21,
22], induce neuronal and cognitive restoration mechanisms to treat metabolic syndrome [
13], affect the growth of protozoan parasites and bacteria [
23,
24,
25,
26], or show antitumor activity [
27,
28]. For example, the compound Mg(H
2O)
6(C
4N
2H
7)
4V
10O
28·4H
2O demonstrated dose-dependent antiproliferative activity on human cancer cells U87, IGR39, and MDA-MB-231 [
29].
Previous works with Adenine and Cytosine have shown a hydrogen bond interaction with the decavanadate anion [
6,
30,
31]. Thus, to obtain new bioactive compounds based on the decavanadate cluster, one organic ligand with potential biological activity was chosen. Here, we report the deployment of 2-aminopyrimidine, which is susceptible to protonation and can interact with the decavanadate anion. Recently, structures formed by decavanadate and ligands with nitrogenous groups with promising antidiabetic and anticancer properties have been published [
22,
29,
32,
33,
34]. In addition, within the family of N-heterocyclic compounds, pyrimidines and their derivatives are an important class of compounds in medicinal chemistry [
35,
36,
37].
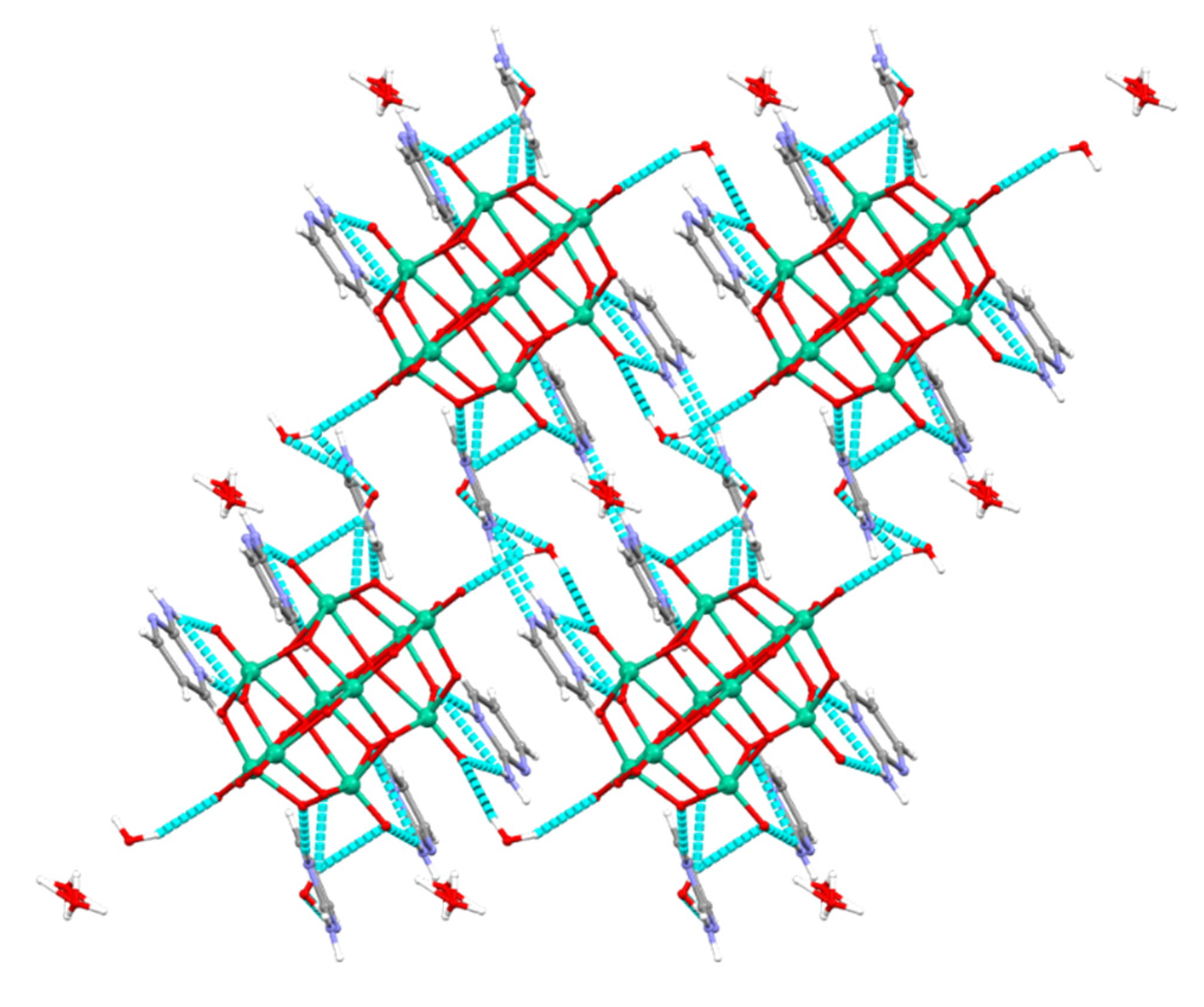
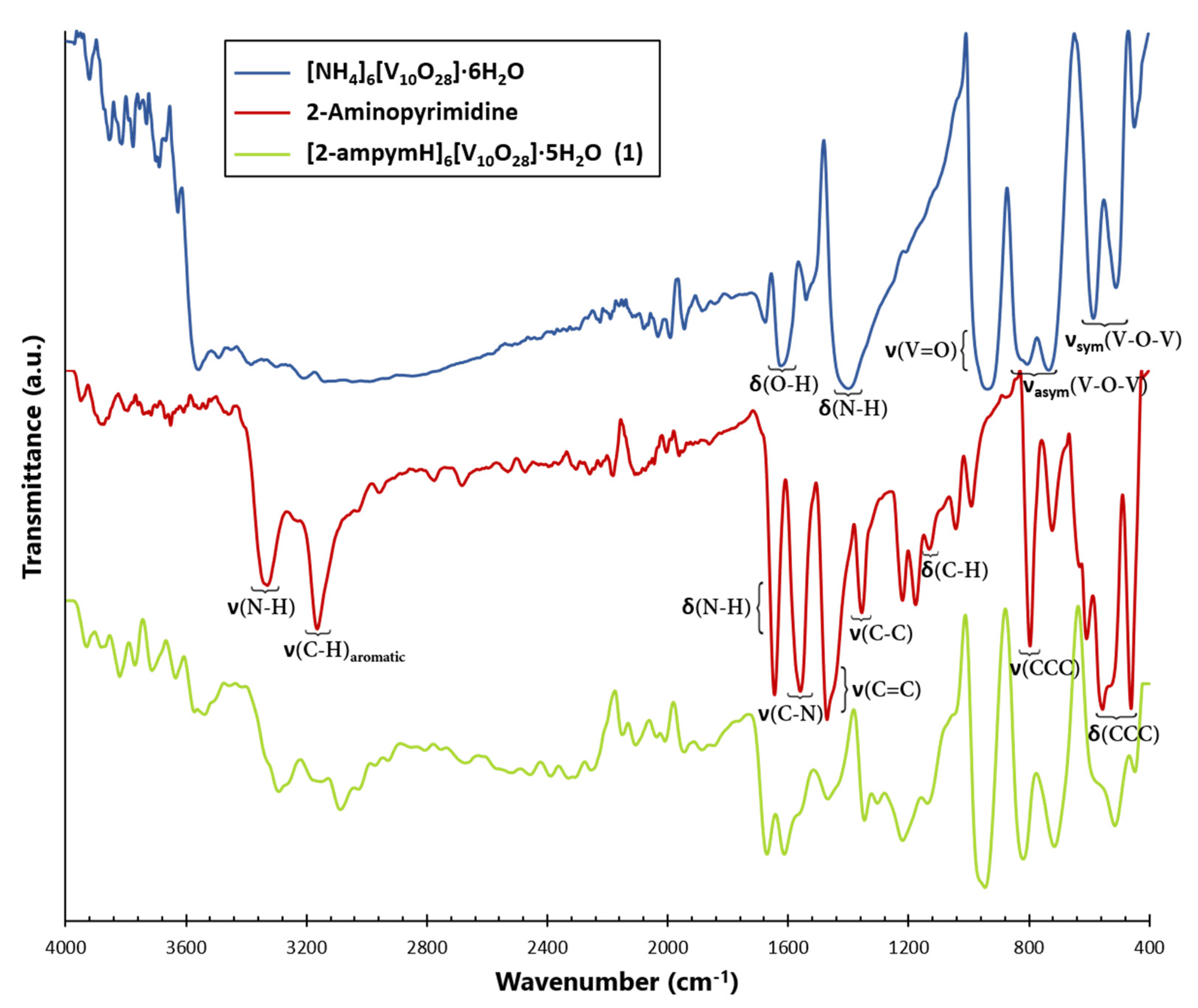
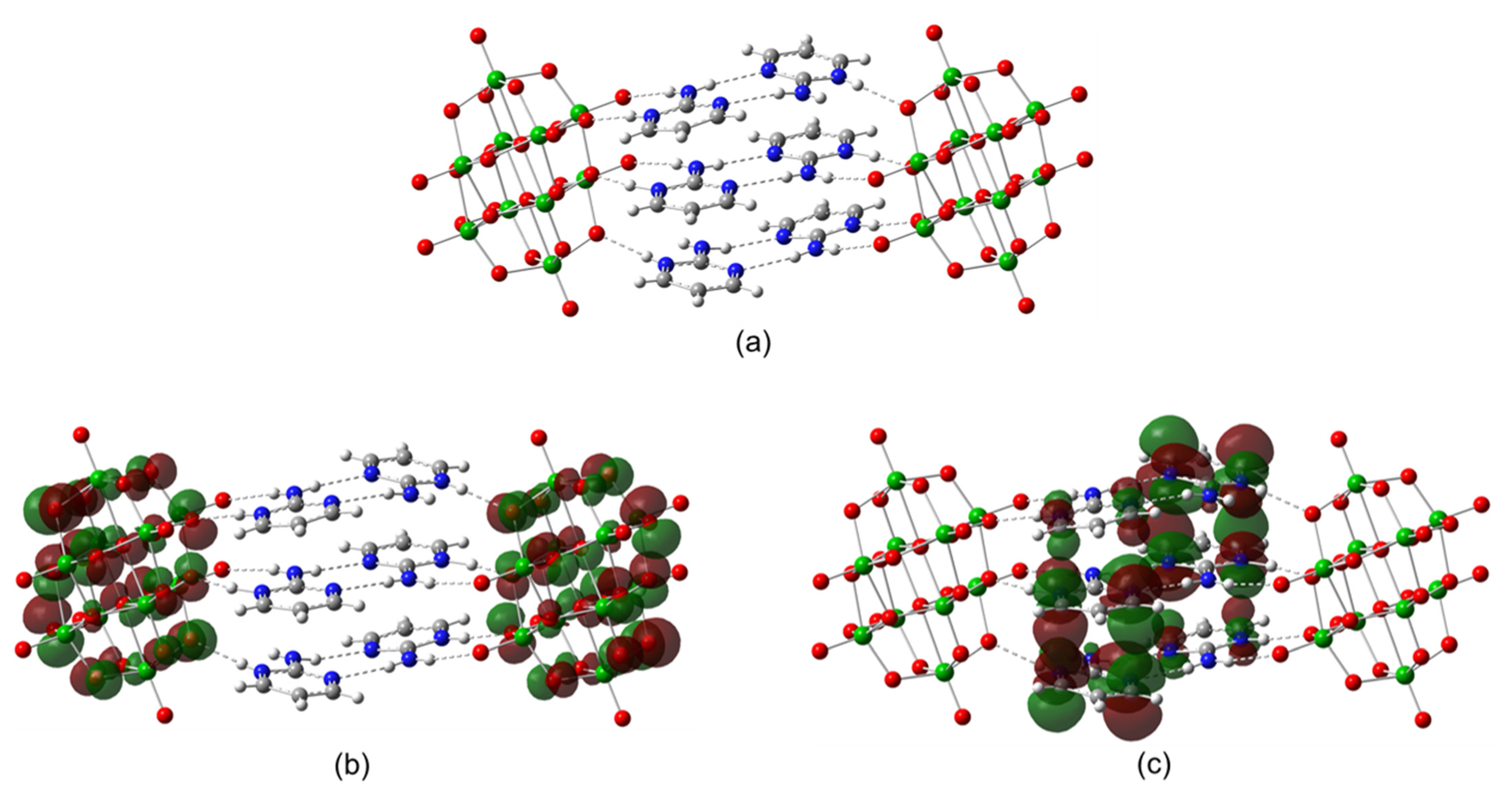
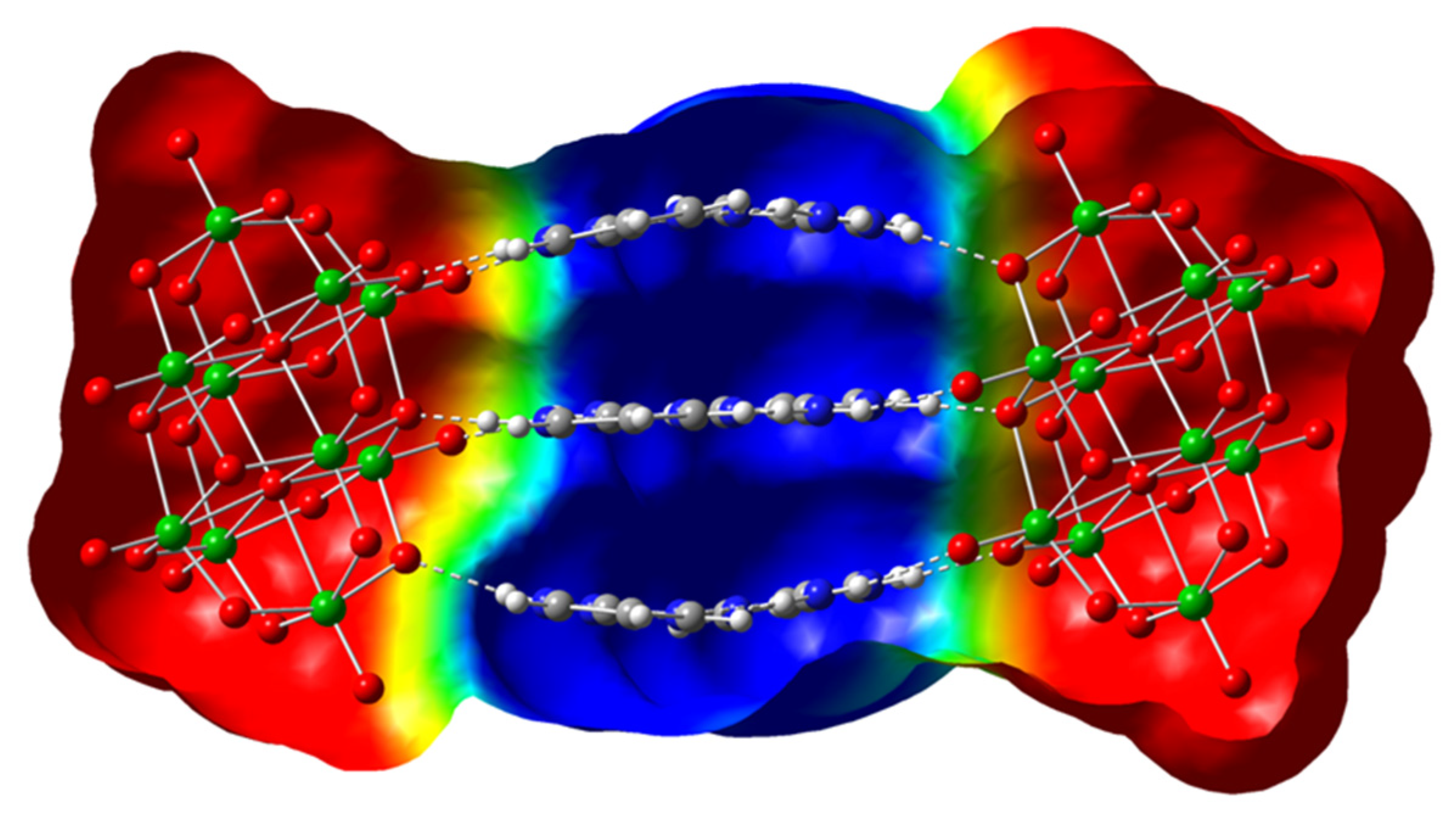
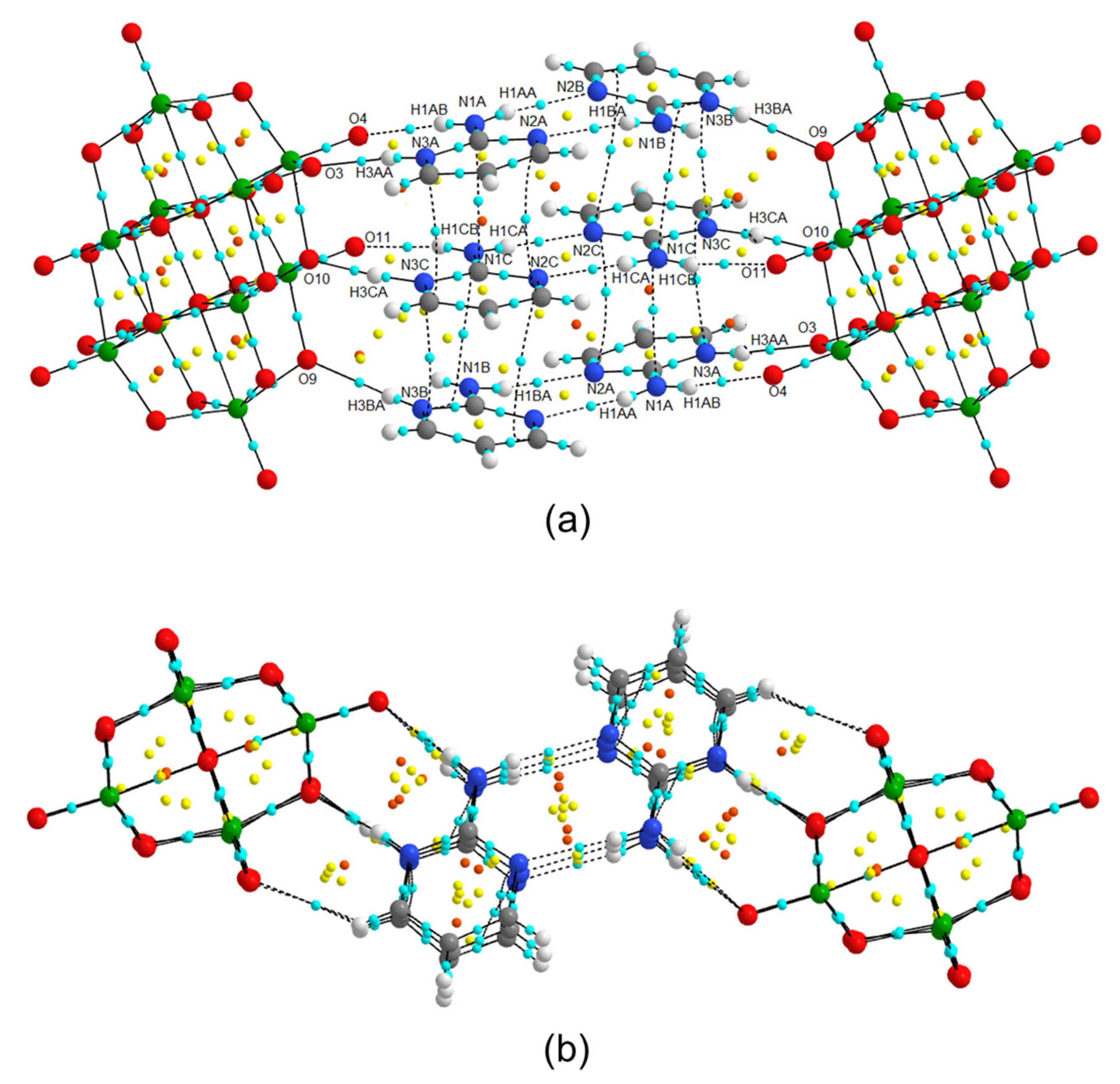
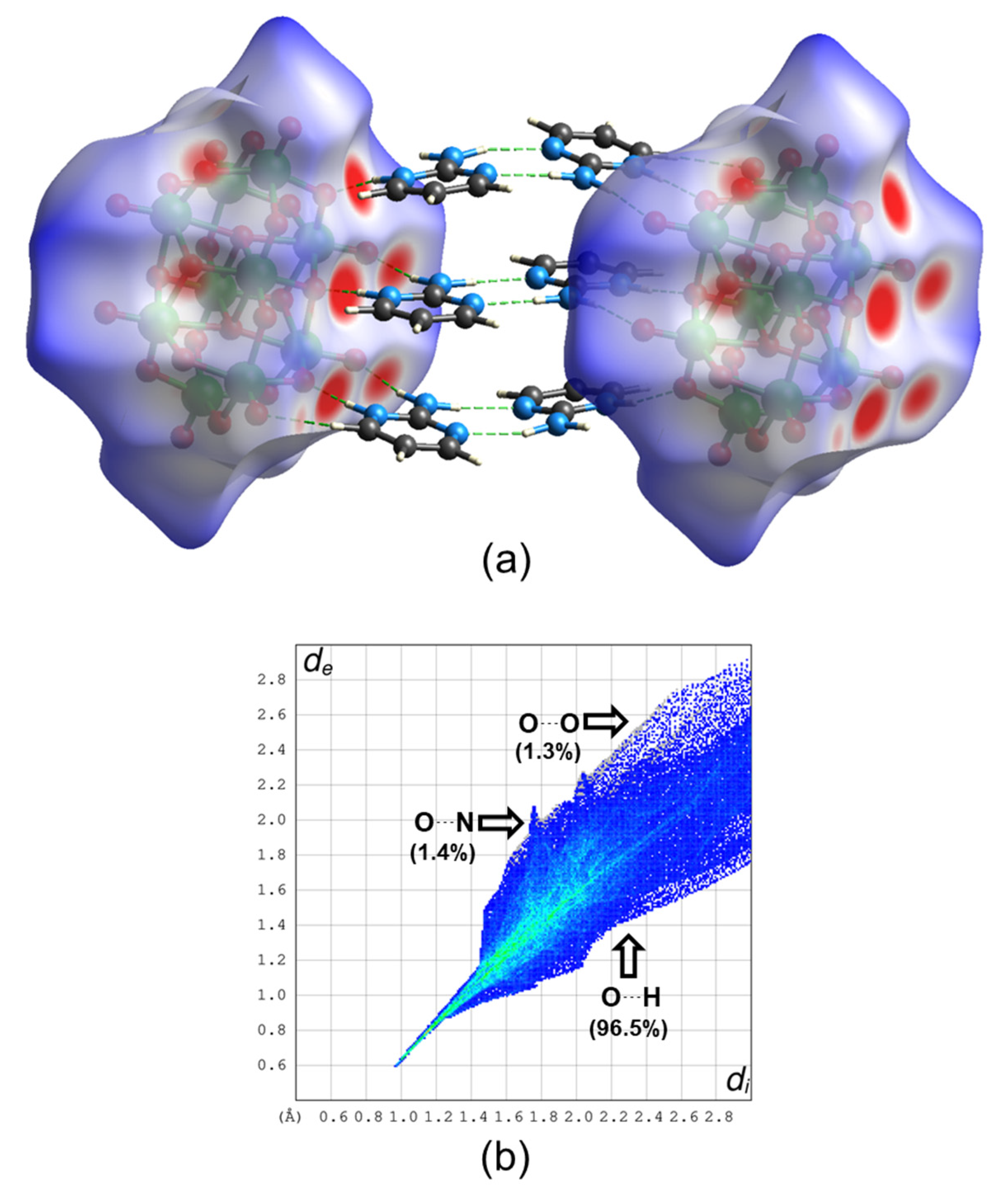
In this way, a new member of a family of compounds based on decavanadate was obtained. Decavanadate anion interacts with a 2-aminopyrimidine ligand to afford a crystalline compound with the formula [2-ampymH]6[V10O28]·5H2O (1). The structural characterization of the compound was carried out by elemental analysis, infrared spectroscopy, thermogravimetric analysis, and single-crystal X-ray diffraction. In addition, the compound was studied using Density Functional Theory (DFT) computational methods. The frontier molecular orbitals and global reactivity indexes were analyzed for showing interesting characteristics of the donor-acceptor interactions. The insights about the compounds’ reactivity were corroborated by analyzing the non-covalent interactions using the AIM approach.
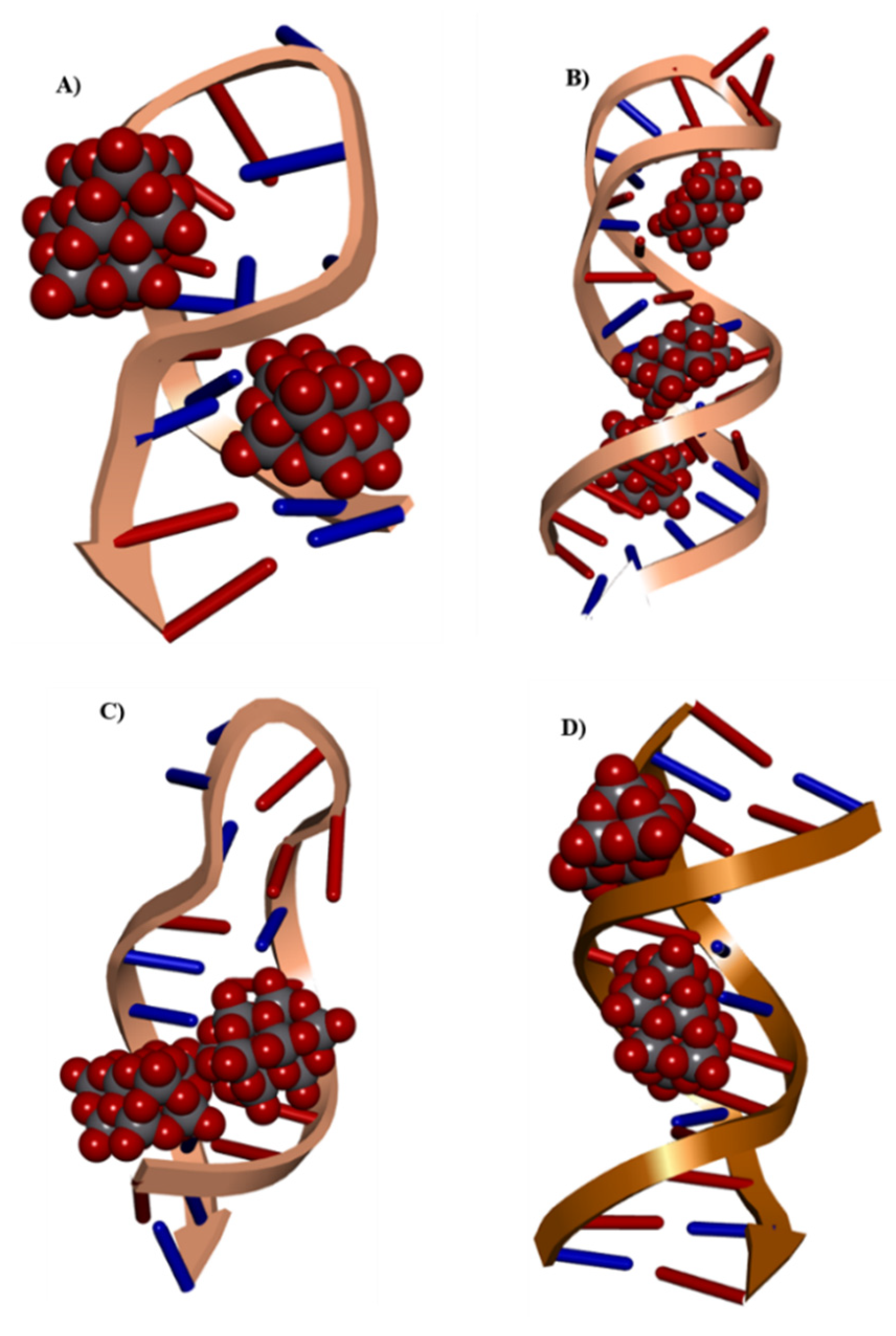
On the other hand, since Sciortino et al. [
38] recently published the interaction of decavanadate with G-actin protein with docking calculations, in this work, docking studies using small RNA and DNA molecules were used to test the hypothesis that the attributed anticancer activity of decavanadate could be due to interaction with these critical molecules. Structurally, the compound has a set of hydrogen bonds and π–π interactions resembling those found in DNA/RNA molecules, opening the field of POM to RNA interactions as potential target molecules for cancer treatment.
3. Discussion
Every year, the global prevalence of cancer rises, as does the resistance to current chemotherapeutic agents like cisplatin; nowadays, one of the main goals of the pharmaceutical industry is the development of more effective drugs for the treatment of cancer [
55,
56].
This work presents a new structure based on the decavanadate cluster and the organic ligand, 2-aminopyrimidine. In general, pyrimidines are one of the most bioactive classes of compounds, with a wide range of biological activities, including in vitro antiviral, diuretic, antitumor, anti-HIV, and cardiovascular effects. Furthermore, pyrimidine moiety is present in the nucleobases that act as building blocks of nucleic acid, DNA or RNA, thymine, cytosine, and uracil, which might be one possible reason for the vast medicinal applications of pyrimidine-derived compounds [
57]. Within the pyrimidines, the aminopyrimidine scaffold is present in the structure of a wide variety of natural and synthetic products, such as Thiamine (vitamin B1), Meridianins (a class of marine alkaloids), or Imatinib, a drug used against leukemia, which displays biological activities such as neuroprotection, antibiotic, antidiabetic, anti-Alzheimer, and anticancer. Among the large family of aminopyrimidines, the 2-aminopyrimidine isomer is the most studied, mainly because of its versatility as a starting material for synthesizing many other bioactive compounds [
55].
Vanadium is an essential element, and it is important to keep the average vanadium concentration in the human body at around 0.3 μM by supplementation via food and drinking water. In the organism, vanadium is found primarily in the form of vanadate H
2VO
4− that, due to the structural and chemical similarity with phosphate, is most likely involved in the regulation of phosphate-dependent processes, such as metabolic pathways involving phosphatases and kinases, as well as phosphate metabolism in general. In addition, organic ligands could aid in the modulation of vanadium’s bioavailability, transport, and targeting mechanism, so, nowadays, coordination compounds containing vanadium are gaining in popularity because of their potential in the treatment of diabetes and cancer, leishmaniasis, and HIV [
58]. In light of this, among vanadate compounds, some researchers have pointed out decavanadates as alternative antitumor agents with promising findings in tumor growth inhibition. Although the anticancer activity of decavanadate is more recent and not yet fully understood, it is probably due to the inhibition of different enzymes such as alkaline phosphatases, ectonucleotidases, and P-type ATPases [
56]. One of the first articles published about decavanadate compounds with potential antitumor activity was Na
4Co(H
2O)
6V
10O
28·18H
2O reported by Zhai et al. [
15]. This compound in vitro displays higher inhibitory activity to human liver cancer (SMMC-7721) and ovary cancer (SK-OV-3) cell lines than 5-fluorouracil, the antitumoral drug clinically used, while in vivo
, it can decrease liver tumor mass in rats. Shortly after, in 2010, Li et al. synthesized two decavanadates compounds with organic ligands that can inhibit proliferation of human lung (A549) and murine leukemia (P388) tumor cell lines in vitro: (H
2tmen)
3V
10O
28·6H
2O and (H
2en)
3V
10O
28·2H
2O [
27]. It is important to highlight that the first compound contains four methyl (–CH
3) substituents in the cation moiety. Their presence may enhance the lipophilic effect of this compound, which increases its penetration through the lipid bilayer of the cell membrane, thus showing higher inhibitory activity than the second compound. After that, a few more articles based on decavanadates compounds with potential antitumor activity have been published up to the present [
29,
53,
59,
60,
61].
Since hydrogen bonds naturally occur between nucleobases and are of great biological importance for DNA and RNA structures, we chose the 2-aminopyrimidine molecule to obtain a new decavanadate compound with potential anticancer activity. Similar to this organic molecule, some structures with cytosine and decavanadate have been published. In the first compound published by Bošnjaković-Pavlović et al. [
36], cytosine forms dimeric cytosine-cytosinium cations that stabilize the charge of the decavanadate anion. A few years later, the second material of decavanadate with cytosine in the literature was published [
37]. In this compound, all cytosine (C) molecules are protonated, and the supramolecular structure is dominated by C–C and C–Decavanadate hydrogen bonds, as well as π-stacking interaction among heterocyclic rings with a centroid-to-centroid distance similar to that found in DNA and RNA structures. The fact that in both structures, cytosines interact with one another by hydrogen bonds and π–π stacking similarly as in DNA and RNA polymers lead to two hypotheses: (i) polyoxidometalates could be employed as templates or catalysts for base-base linkages, and (ii) base-base pairing was crucial in the early stages of life [
37]. Why nucleobases tend to form this arrangement in DNA and RNA has been studied by Francés-Monerris et al. [
62]. The intra- and inter-strand interactions in Watson–Crick base pairs are of great importance for the thermal stability of the double-helix structure. In fact, in their experiments, upon UV light exposure at a B-DNA arrangement, C–C dimers twist towards a face-to-face arrangement to increase the π-stacking interaction and further promote the photostability of the genomic material. On the other hand, structures containing purine-based ligands show the same fact, as in the (NH
4)
2(C
8H
10N
4O
2)
4[H
4V
10O
28]·2H
2O compound, π–π stacking interactions with an interplanar distance of 3.38 Å exist in the purine rings of caffeine [
63], and in [AdH]
6[V
10O
38]·4H
2O, Adeninium cations form simultaneous hydrogen bond interactions in a ribbon-like geometry as well as π–π interaction between cationic ribbons [
6].
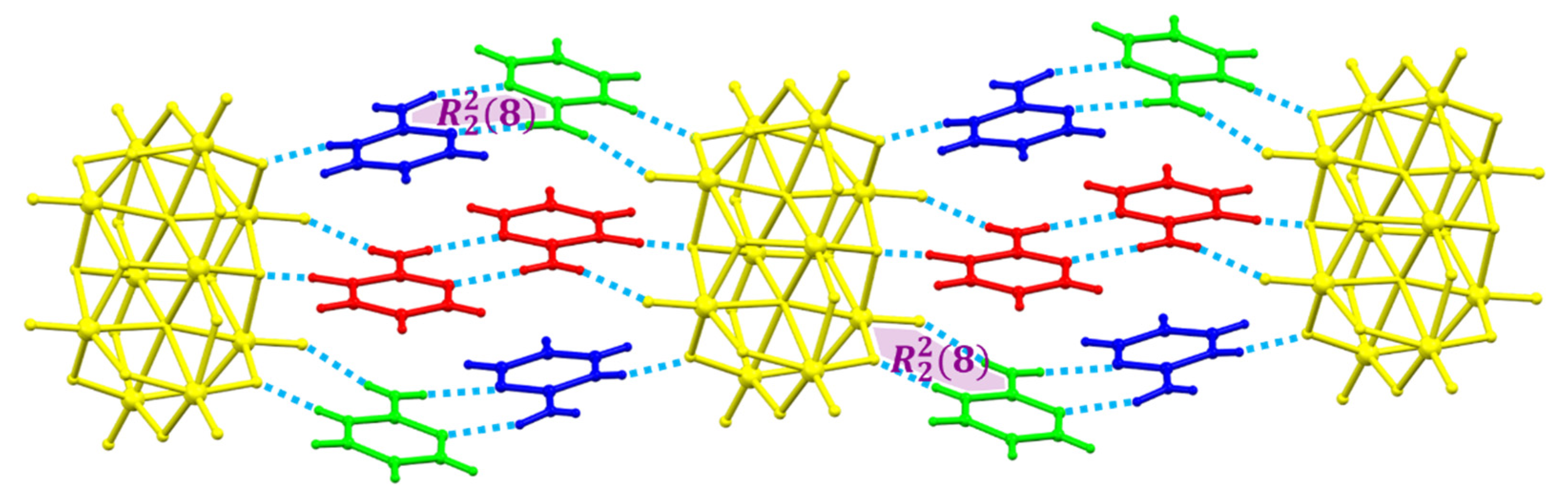
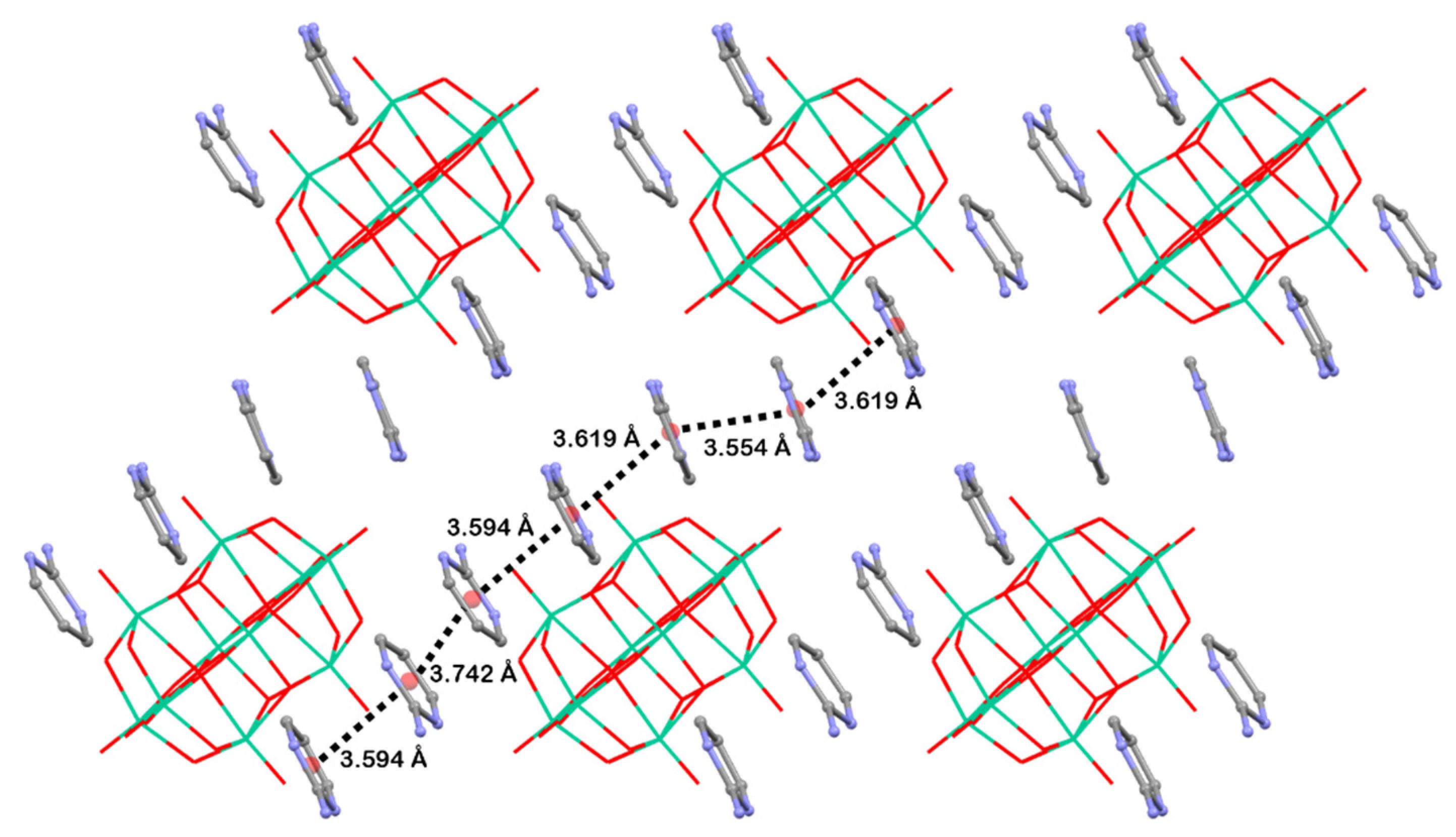
All these facts support the structure of Compound 1 in this paper, since 2-aminopyrimidinium cations are disposed of in the form that they interact with each other by hydrogen bonds and π–π stacking interactions with a centroid-to centroid distance of around 3.6 Å.
Furthermore, to study the potential anticarcinogenic activity of Compound
1, docking tests were performed to analyze the interaction of the decavanadate anion with some types of microRNAs (miRNAs) and DNA molecules. MiRNAs are small, non-coding RNA molecules with a length of 19–25 nucleotides that regulate various target genes. MiRNAs play a role in the cell cycle, differentiation, proliferation, energy metabolism, and immunological response, among other biological activities, regulating around 30% of human genes, with half of these genes being tumor-related. Recent studies have found that miRNAs play an important role in cancer progression, including tumor growth, differentiation, adhesion, apoptosis, invasion, and metastasis [
64,
65]. Within the miRNA family, the upregulations of miR-21, as well as lncRNA (long non-coding RNA), are linked to several types of cancer such as malignant B-cell lymphoma or breast cancer, respectively [
66,
67]. On the other hand, let-7 miRNA (member of the family of let-7 RNA) is known as the keeper of differentiation and has also emerged as a promising therapeutic agent to treat cancer and immune responses [
68,
69]. Thus, it is interesting to carry out experiments to prove this hypothesis, since our preliminary analyses show great affinity of decavanadate for miRNA fragments.
In addition to the possible antitumoral activity that Compound
1 could exhibit, the interesting structure that it shows, along with all the above examples about structures with decavanadate clusters and organic ligands that arrange remembering the DNA or RNA structures, highlights the idea that these polyoxidovanadates could act as templates or catalysts for base-base pairing. Bernal, in 1949, was the first who proposed the important role of clay minerals in the origin of life [
70]. The advantage of these clays could include ordered arrangement, substantial adsorption capacity, UV protection, ability to concentrate organic compounds, and potential to serve as polymerization templates so that clay minerals could have played a key role in chemical evolution and the origin of life [
71]. Intercalation of decavanadate into laminar minerals has already been achieved [
72], so it is possible to concentrate organic compounds with a high capacity for hydrogen bond formation and catalyze their polymerization. Therefore, it will be worthwhile to explore this idea.
Additionally, it is important to mention that recent work from Aureliano’s group about melanoma anticancer activity of a variety of vanadium compounds [
73] and potential anti-SARS-CoV-2 activity of vanadium compounds by Scior et al. [
74], present a promising future for vanadium compounds as metallodrugs.