Investigation of Patient-Specific Maxillofacial Implant Prototype Development by Metal Fused Filament Fabrication (MF3) of Ti-6Al-4V
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of the Implant
2.1.1. Image Processing and Segmentation
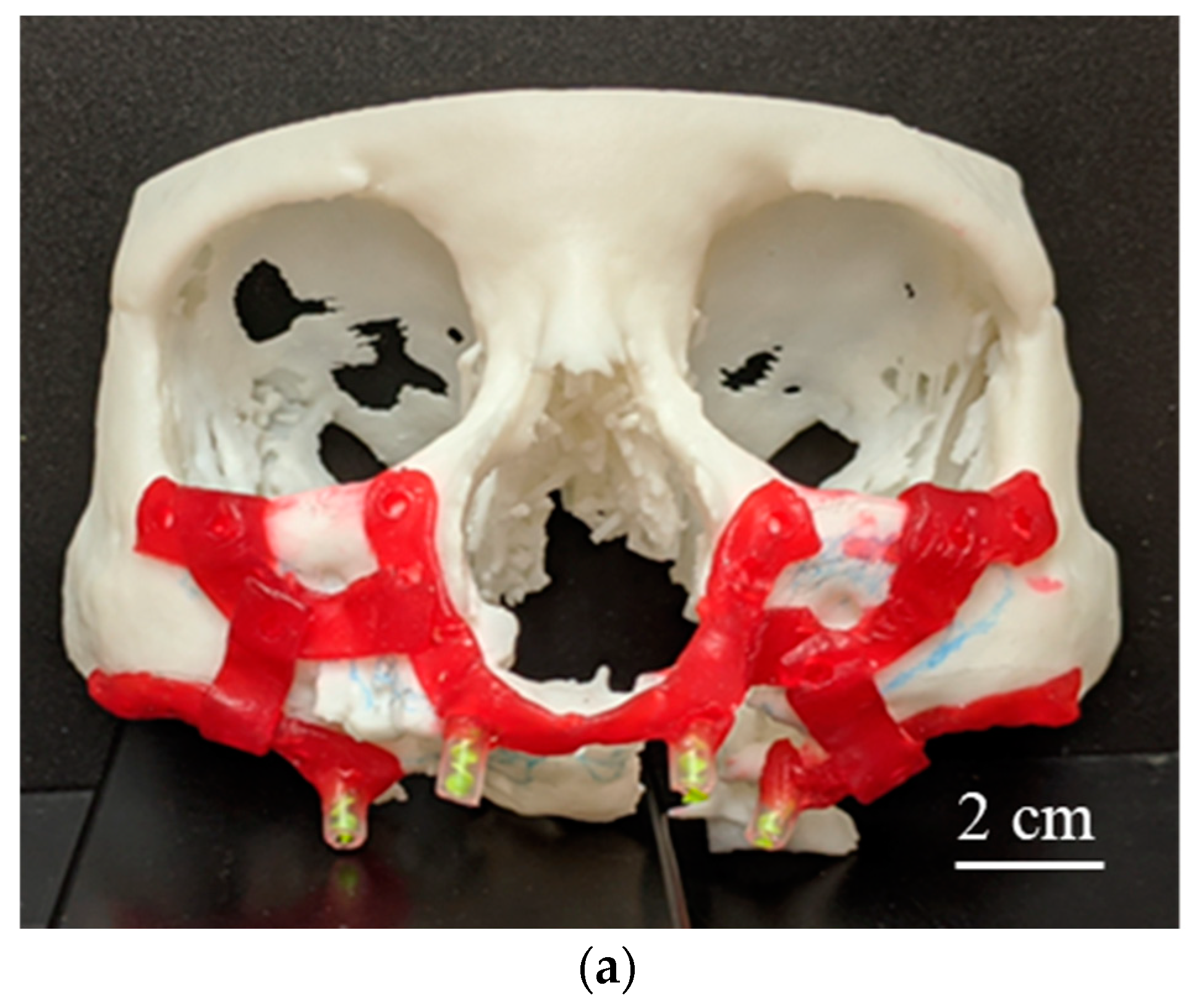
2.1.2. 3D-Printed Physical Anatomical Model
2.1.3. Implant Design Development
2.2. Fabrication of Customized Ti-6Al-4V Alloy Implant Using MF3
2.2.1. Support Structure
2.2.2. Debinding and Sintering
2.2.3. Green and Sintered Parts Characterization
2.3. MF3 Process Simulation
3. Results
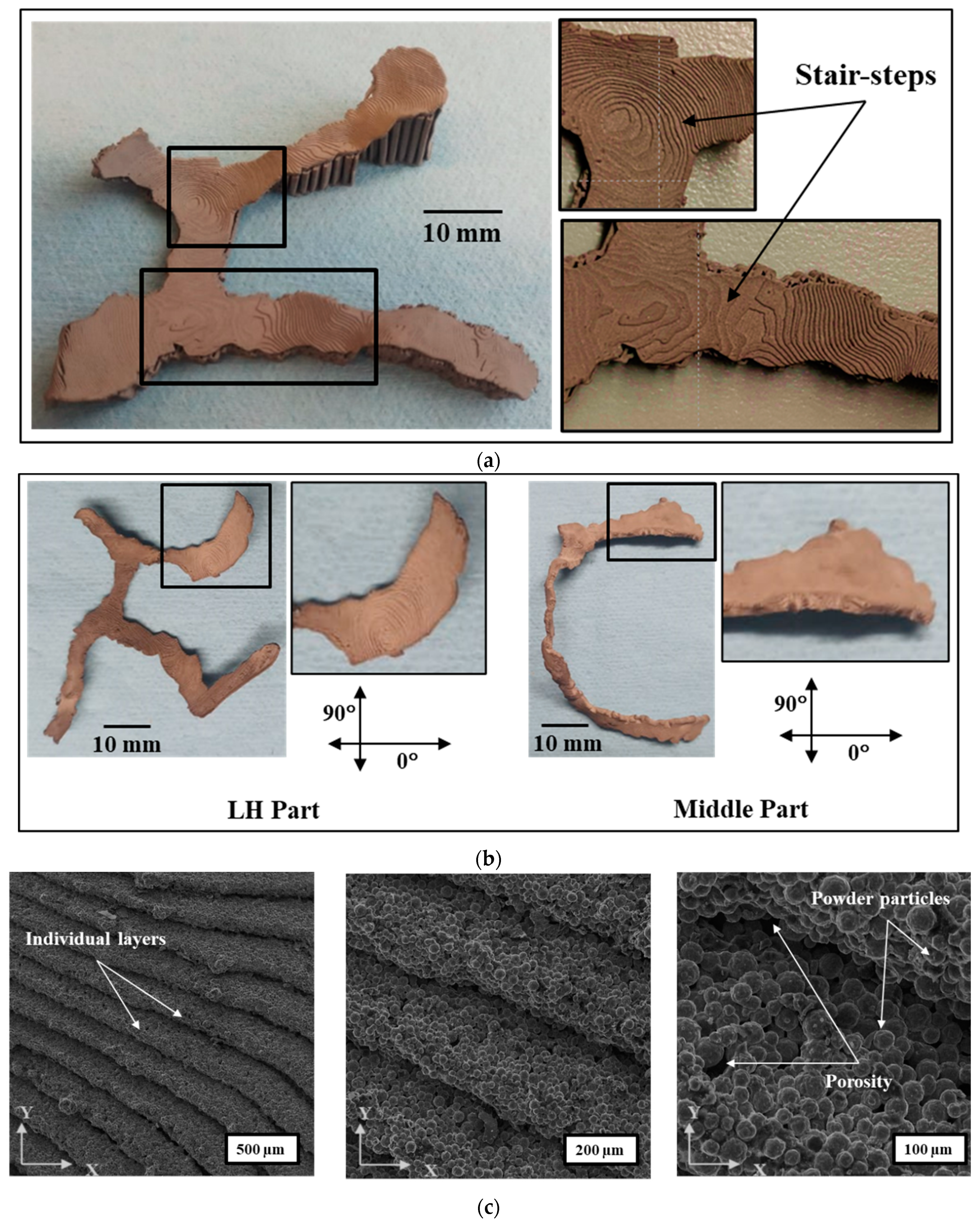
3.1. MF3 Printed Green Parts
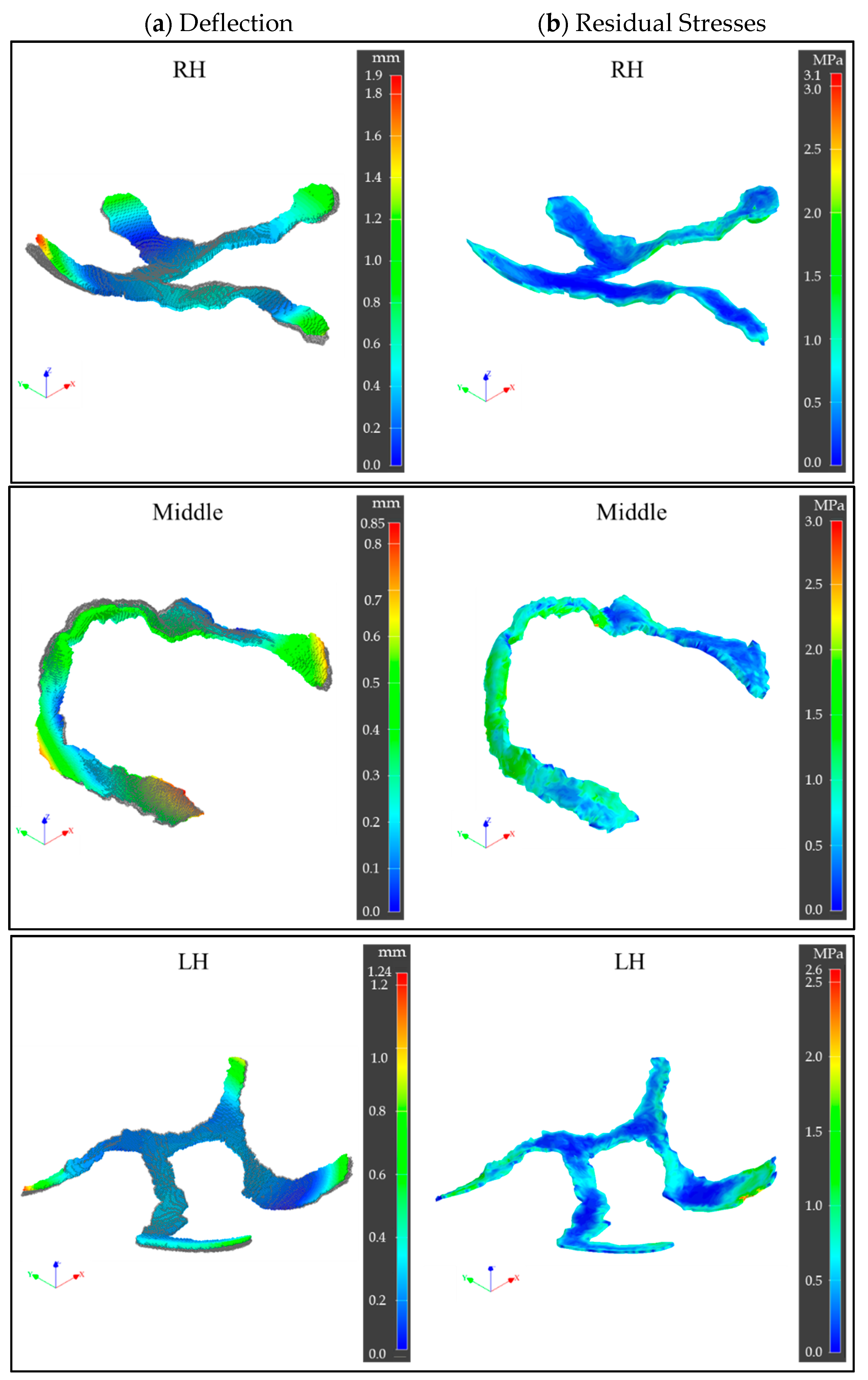
3.2. Printing Process Simulations
3.3. Sintered Ti-6Al-4V Parts Characterization
3.3.1. Surface Topography
3.3.2. Relative Density and Porosity
3.3.3. Metallography
4. Discussions
Future Work
5. Conclusions
- Fabrication of patient-specific custom maxillofacial implant prototypes out of Ti-6Al-4V by MF3 has been found feasible and demonstrated through the experimental study. However, as the design was in a preliminary stage, some critical parts such as holes and mounting posts were not included in this study. Further investigations are still needed to investigate fully equipped prototypes for a real assessment and final validation.
- A specific digital workflow is required to convert the patient’s CBCT data into a 3D printable format that made additive manufacturing of the anatomical model and the maxillofacial implant prototypes possible.
- MF3 printing with support structures was reported for the first time. Optimal support structures were required in MF3 for custom implant geometries to ensure geometric fidelity not only during printing but also debinding and sintering processes.
- MF3 process simulation estimated maximum deflections of 0.9–1.9 mm and maximum residual stresses of ~3 MPa in printed green parts. However, the accuracy of prediction would be affected by the absence of support structures in simulations as opposed to experimental printing.
- The relative density (bulk density-based) of the Middle part was found to be 81% indicating the total porosity of 19% including 6% closed porosity and 13% open interconnected porosity that would provide anchor sites to the bone tissue and promotes accelerated osseointegration.
- Stair-step effects and lack of diffusion between layers contributed to surface roughness at the macro scale, whereas powder particles and porosity within a layer resulted at the micro-scale. LH part showed higher roughness (Ra: ~23 µm) in 0° than that of Middle part (Ra: 13 µm), while in 90° both parts showed the same results (Ra: ~13 µm). The difference in part geometry and orientation on the print bed led to different surface angles and toolpath, hence the variation in surface roughness, accordingly. Higher surface roughness was observed with a higher surface angle with the build plate.
- The hardness of 6.5 ± 0.8 HRC was observed in the Ti-6Al-4V implants printed by MF3 as opposed to 45 ± 10 HRC in EBM and SLM.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tamimi, F.; Torres, J.; Al-Abedalla, K.; Lopez-Cabarcos, E.; Alkhraisat, M.H.; Bassett, D.; Gbureck, U.; Barralet, J.E. Osseointegration of dental implants in 3D-printed synthetic onlay grafts customized according to bone metabolic activity in recipient site. Biomaterials 2014, 35, 5436–5445. [Google Scholar] [CrossRef]
- Osman, R.B.; van der Veen, A.J.; Huiberts, D.; Wismeijer, D.; Alharbi, N. 3D-printing zirconia implants; a dream or a reality? An in-vitro study evaluating the dimensional accuracy, surface topography and mechanical properties of printed zirconia implant and discs. J. Mech. Behav. Biomed. Mater. 2017, 75, 521–528. [Google Scholar] [CrossRef]
- Oliveira, T.T.; Reis, A. Fabrication of dental implants by the additive manufacturing method: A systematic review. J. Prosthet. Dent. 2019, 122, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Titsinides, S.; Agrogiannis, G.; Karatzas, T. Bone grafting materials in dentoalveolar reconstruction: A comprehensive review. Jpn. Dent. Sci. Rev. 2019, 55, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Batool, F.; Strub, M.; Petit, C.; Bugueno, I.M.; Bornert, F.; Clauss, F.; Huck, O.; Kuchler-Bopp, S.; Benkirane-Jessel, N. Periodontal tissues, maxillary jaw bone, and tooth regeneration approaches: From animal models analyses to clinical applications. Biomaterials 2018, 8, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, E.; Van Roekel, N.B.; Desjardins, R.P.; Tolman, D.E. Prosthetic-surgical reconstruction of the severely resorbed maxilla with iliac bone grafting and tissue-integrated prostheses. Int. J. Oral Maxillofac. Implant. 1987, 2, 39–55. [Google Scholar]
- Gellrich, N.-C.; Zimmerer, R.M.; Spalthoff, S.; Jehn, P.; Pott, P.-C.; Rana, M.; Rahlf, B. A customised digitally engineered solution for fixed dental rehabilitation in severe bone deficiency: A new innovative line extension in implant dentistry. J. Cranio-Maxillofac. Surg. 2017, 45, 1632–1638. [Google Scholar] [CrossRef]
- Mangano, C.; Bianchi, A.; Mangano, F.G.; Dana, J.; Colombo, M.; Solop, I.; Admakin, O. Custom-made 3D printed subperiosteal titanium implants for the prosthetic restoration of the atrophic posterior mandible of elderly patients: A case series. 3D Print. Med. 2020, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Sokac, M.; Budak, I.; Miljanovic, D.; Santosi, Z.; Vukelic, D. Computer-aided modeling and additive manufacturing fabrication of patient-specific mandibular implant. Lékař Tech. Clin. Technol. 2018, 48, 113–117. [Google Scholar]
- Fry, R.R.; Gargya, I.; Goyal, S.; Chawla, J.P.S.; Pandher, P.K.; Dhaliwal, G.; Ghotra, P.S. Additive manufacturing—An enigma: The future of oral & maxillofacial surgery. IOSR J. Dent. Med. Sci. 2016, 15, 78–83. [Google Scholar] [CrossRef]
- Dincă, L.L.; Banu, A.; Vişan, A. Additive manufacturing in maxillofacial reconstruction. MATEC Web Conf. 2017, 137, 2001. [Google Scholar] [CrossRef] [Green Version]
- Cohen, D.J.; Cheng, A.; Kahn, A.; Aviram, M.; Whitehead, A.J.; Hyzy, S.L.; Clohessy, R.M.; Boyan, B.D.; Schwartz, Z. Novel osteogenic Ti-6Al-4V device for restoration of dental function in patients with large bone deficiencies: Design, development and implementation. Sci. Rep. 2016, 6, 20493. [Google Scholar] [CrossRef] [Green Version]
- Jardini, A.L.; Larosa, M.A.; Filho, R.M.; Zavaglia, C.A.D.C.; Bernardes, L.F.; Lambert, C.S.; Calderoni, D.R.; Kharmandayan, P. Cranial reconstruction: 3D biomodel and custom-built implant created using additive manufacturing. J. Cranio-Maxillofac. Surg. 2014, 42, 1877–1884. [Google Scholar] [CrossRef]
- Stoor, P.; Suomalainen, A.; Mesimäki, K.; Kontio, R. Rapid prototyped patient specific guiding implants in critical mandibular reconstruction. J. Cranio-Maxillofac. Surg. 2017, 45, 63–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naros, A.; Weise, H.; Tilsen, F.; Hoefert, S.; Naros, G.; Krimmel, M.; Reinert, S.; Polligkeit, J. Three-dimensional accuracy of mandibular reconstruction by patient-specific pre-bent reconstruction plates using an “in-house” 3D-printer. J. Cranio-Maxillofac. Surg. 2018, 46, 1645–1651. [Google Scholar] [CrossRef]
- Gonzalez-Gutierrez, J.; Cano, S.; Schuschnigg, S.; Kukla, C.; Sapkota, J.; Holzer, C. Additive manufacturing of metallic and ceramic components by the material extrusion of highly-filled polymers: A review and future perspectives. Materials 2018, 11, 840. [Google Scholar] [CrossRef] [Green Version]
- Sing, S.L.; An, J.; Yeong, W.Y.; Wiria, F.E. Laser and electron-beam powder-bed additive manufacturing of metallic implants: A review on processes, materials and designs. J. Orthop. Res. 2016, 34, 369–385. [Google Scholar] [CrossRef]
- Bai, L.; Gong, C.; Chen, X.; Sun, Y.; Zhang, J.; Cai, L.; Zhu, S.; Xie, S.Q. Additive manufacturing of customized metallic orthopedic implants: Materials, structures, and surface modifications. Metals 2019, 9, 1004. [Google Scholar] [CrossRef] [Green Version]
- Cohen, D.J.; Cheng, A.; Sahingur, K.; Clohessy, R.M.; Hopkins, L.B.; Boyan, B.D.; Schwartz, Z. Performance of laser sintered Ti-6Al-4V implants with bone-inspired porosity and micro/nanoscale surface roughness in the rabbit femur. Biomed. Mater. 2017, 12, 025021. [Google Scholar] [CrossRef] [PubMed]
- Kolken, H.; Lietaert, K.; van der Sloten, T.; Pouran, B.; Meynen, A.; Van Loock, G.; Weinans, H.; Scheys, L.; Zadpoor, A. Mechanical performance of auxetic meta-biomaterials. J. Mech. Behav. Biomed. Mater. 2020, 104, 103658. [Google Scholar] [CrossRef]
- Szost, B.A.; Terzi, S.; Martina, F.; Boisselier, D.; Prytuliak, A.; Pirling, T.; Hofmann, M.; Jarvis, D.J. A comparative study of additive manufacturing techniques: Residual stress and microstructural analysis of CLAD and WAAM printed Ti-6Al-4V components. Mater. Des. 2016, 89, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhang, Z.; Chen, X.; Zhang, C.; Zhang, G.; Xu, Z. Design and manufacture of customized dental implants by using reverse engineering and selective laser melting technology. J. Prosthet. Dent. 2014, 112, 1088–1095.e1. [Google Scholar] [CrossRef] [PubMed]
- Hyzy, S.L.; Cheng, A.; Cohen, D.J.; Yatzkaier, G.; Whitehead, A.J.; Clohessy, R.M.; Gittens, R.A.; Boyan, B.D.; Schwartz, Z. Novel hydrophilic nanostructured microtexture on direct metal laser sintered Ti-6Al-4V surfaces enhances osteoblast responsein vitroand osseointegration in a rabbit model. J. Biomed. Mater. Res. Part A 2016, 104, 2086–2098. [Google Scholar] [CrossRef]
- Kok, Y.; Tan, X.; Wang, P.; Nai, M.; Loh, N.; Liu, E.; Tor, S.B. Anisotropy and heterogeneity of microstructure and mechanical properties in metal additive manufacturing: A critical review. Mater. Des. 2018, 139, 565–586. [Google Scholar] [CrossRef]
- Lewandowski, J.; Seifi, M. Metal additive manufacturing: A review of mechanical properties. Annu. Rev. Mater. Res. 2016, 46, 151–186. [Google Scholar] [CrossRef] [Green Version]
- Campioni, I.; Gupta, N. Ti6Al4V mandibular devices by additive manufacturing: Assessment of as-built quality. Med. Devices Sens. 2021, 4, 10153. [Google Scholar] [CrossRef]
- Singh, P.; Shaikh, Q.; Balla, V.K.; Atre, S.V.; Kate, K.H. Estimating powder-polymer material properties used in design for metal fused filament fabrication (DfMF3). JOM 2020, 72, 485–495. [Google Scholar] [CrossRef]
- Singh, P.; Balla, V.K.; Tofangchi, A.; Atre, S.V.; Kate, K.H. Printability studies of Ti-6Al-4V by metal fused filament fabrication (MF3). Int. J. Refract. Met. Hard Mater. 2020, 91, 105249. [Google Scholar] [CrossRef]
- Singh, P.; Balla, V.K.; Gokce, A.; Atre, S.V.; Kate, K.H. Additive manufacturing of Ti-6Al-4V alloy by metal fused filament fabrication (MF3): Producing parts comparable to that of metal injection molding. Prog. Addit. Manuf. 2021, 1–14. [Google Scholar] [CrossRef]
- Shaikh, M.Q.; Singh, P.; Kate, K.H.; Freese, M.; Atre, S.V. Finite element-based simulation of metal fused filament fabrication process: Distortion prediction and experimental verification. J. Mater. Eng. Perform. 2021, 30, 5135–5149. [Google Scholar] [CrossRef]
- Shaikh, M.Q.; Lavertu, P.-Y.; Kate, K.H.; Atre, S.V. Process sensitivity and significant parameters investigation in metal fused filament fabrication of Ti-6Al-4V. J. Mater. Eng. Perform. 2021, 30, 5118–5134. [Google Scholar] [CrossRef]
- Shaikh, M.Q.; Graziosi, S.; Atre, S.V. Supportless printing of lattice structures by metal fused filament fabrication (MF3) of Ti-6Al-4V: Design and analysis. Rapid Prototyp. J. 2021, 27, 1408–1422. [Google Scholar] [CrossRef]
- Yau, Y.Y.; Arvier, J.F.; Barker, T.M. Maxillofacial biomodelling—Preliminary result. Br. Inst. Radiol. 2014, 68, 809. [Google Scholar] [CrossRef]
- Thomas, D.J.; Azmi, M.A.B.M.; Tehrani, Z.A. 3D additive manufacture of oral and maxillofacial surgical models for preoperative planning. Int. J. Adv. Manuf. Technol. 2014, 71, 1643–1651. [Google Scholar] [CrossRef]
- Suomalainen, A.; Stoor, P.; Mesimäki, K.; Kontio, R.K. Rapid prototyping modelling in oral and maxillofacial surgery: A two year retrospective study. J. Clin. Exp. Dent. 2015, 7, e605–e612. [Google Scholar] [CrossRef] [Green Version]
- Singh, P. Materials-Processing Relationships for Metal Fused Filament Fabrication of Ti-6Al-4V Alloy. Ph.D. Thesis, University of Louisville, Louisville, KY, USA, 2020. [Google Scholar]
- Shalabi, M.; Gortemaker, A.; Hof, M.V.; Jansen, J.; Creugers, N. Implant surface roughness and bone healing: A systematic review. J. Dent. Res. 2006, 85, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Kieswetter, K.; Schwartz, Z.; Dean, D.; Boyan, B. The role of implant surface characteristics in the healing of bone. Crit. Rev. Oral Biol. Med. 1996, 7, 329–345. [Google Scholar] [CrossRef] [Green Version]
- Muallah, D.; Sembdner, P.; Holtzhausen, S.; Meissner, H.; Hutsky, A.; Ellmann, D.; Assmann, A.; Schulz, M.; Lauer, G.; Kroschwald, L. Adapting the pore size of individual, 3D-printed CPC scaffolds in maxillofacial surgery. J. Clin. Med. 2021, 10, 2654. [Google Scholar] [CrossRef]
- Murr, L.; Quinones, S.; Gaytan, S.; Lopez, M.; Rodela, A.; Martinez, E.; Hernandez, D.; Medina, F.; Wicker, R. Microstructure and mechanical behavior of Ti-6Al-4V produced by rapid-layer manufacturing, for biomedical applications. J. Mech. Behav. Biomed. Mater. 2009, 2, 20–32. [Google Scholar] [CrossRef]
- Sun, Q.; Rizvi, G.M.; Bellehumeur, C.T.; Gu, P. Effect of Processing Conditions on The Bonding Quality of FDM Polymer Filaments. Rapid Prototyp. J. 2008, 14, 72–80. [Google Scholar] [CrossRef]
- Tunchel, S.; Blay, A.; Kolerman, R.; Mijiritsky, E.; Shibli, J.A. 3D printing/additive manufacturing single titanium dental implants: A prospective multicenter study with 3 years of follow-up. Int. J. Dent. 2016, 2016, 8590971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeVasConCellos, P.; Balla, V.K.; Bose, S.; Fugazzi, R.; Dernell, W.S.; Bandyopadhyay, A. Patient specific implants for amputation prostheses: Design, manufacture and analysis. Vet. Comp. Orthop. Traumatol. 2012, 25, 286–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öhman, C.; Dall’Ara, E.; Baleani, M.; Jan, S.V.S.; Viceconti, M. The effects of embalming using a 4% formalin solution on the compressive mechanical properties of human cortical bone. Clin. Biomech. 2008, 23, 1294–1298. [Google Scholar] [CrossRef] [Green Version]








| Process Parameters | Layer Thickness (mm) | Bead Width (mm) | Extrusion Temperature (°C) | Build Plate Temperature (°C) | Extrusion Multiplier | Printing Speed (mm/s) | Toolpath |
|---|---|---|---|---|---|---|---|
| Settings | 0.1 | 0.48 | 240 | 65 | 1 | 5 | Concentric |
| Part | Measurement Angle | Ra (µm) |
|---|---|---|
| LH | 0° | 23.3 ± 1.0 |
| 90° | 12.9 ± 1.2 | |
| Middle | 0° | 13.5 ± 1.0 |
| 90° | 12.7 ± 0.7 |
| Archimedes Density (g/cc) | Relative Density (%) (AD-Based) | Bulk Density (g/cc) | Relative Density (%) (BD-Based) | |
|---|---|---|---|---|
| Middle part | 4.18 | 94.3 | 3.60 | 81.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaikh, M.Q.; Nath, S.D.; Akilan, A.A.; Khanjar, S.; Balla, V.K.; Grant, G.T.; Atre, S.V. Investigation of Patient-Specific Maxillofacial Implant Prototype Development by Metal Fused Filament Fabrication (MF3) of Ti-6Al-4V. Dent. J. 2021, 9, 109. https://0-doi-org.brum.beds.ac.uk/10.3390/dj9100109
Shaikh MQ, Nath SD, Akilan AA, Khanjar S, Balla VK, Grant GT, Atre SV. Investigation of Patient-Specific Maxillofacial Implant Prototype Development by Metal Fused Filament Fabrication (MF3) of Ti-6Al-4V. Dentistry Journal. 2021; 9(10):109. https://0-doi-org.brum.beds.ac.uk/10.3390/dj9100109
Chicago/Turabian StyleShaikh, Mohammad Qasim, Subrata Deb Nath, Arulselvan Arumugam Akilan, Saleh Khanjar, Vamsi Krishna Balla, Gerald Thomas Grant, and Sundar Vedanarayanan Atre. 2021. "Investigation of Patient-Specific Maxillofacial Implant Prototype Development by Metal Fused Filament Fabrication (MF3) of Ti-6Al-4V" Dentistry Journal 9, no. 10: 109. https://0-doi-org.brum.beds.ac.uk/10.3390/dj9100109






