Effect of Butyric Acid in the Proliferation and Migration of Junctional Epithelium in the Progression of Periodontitis: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Proliferation and Inhibition Assay
2.3. Migration Assay
2.4. Cell Attachment Assay
2.5. Real-Time Quantitative Reverse-Transcription Polymerase Chain Reaction
2.6. Gene and Promoter Expression Analysis
2.7. Gene Ontology (GO) Term Enrichment Analysis of CAGE Data
2.8. Statistical Analysis
3. Results
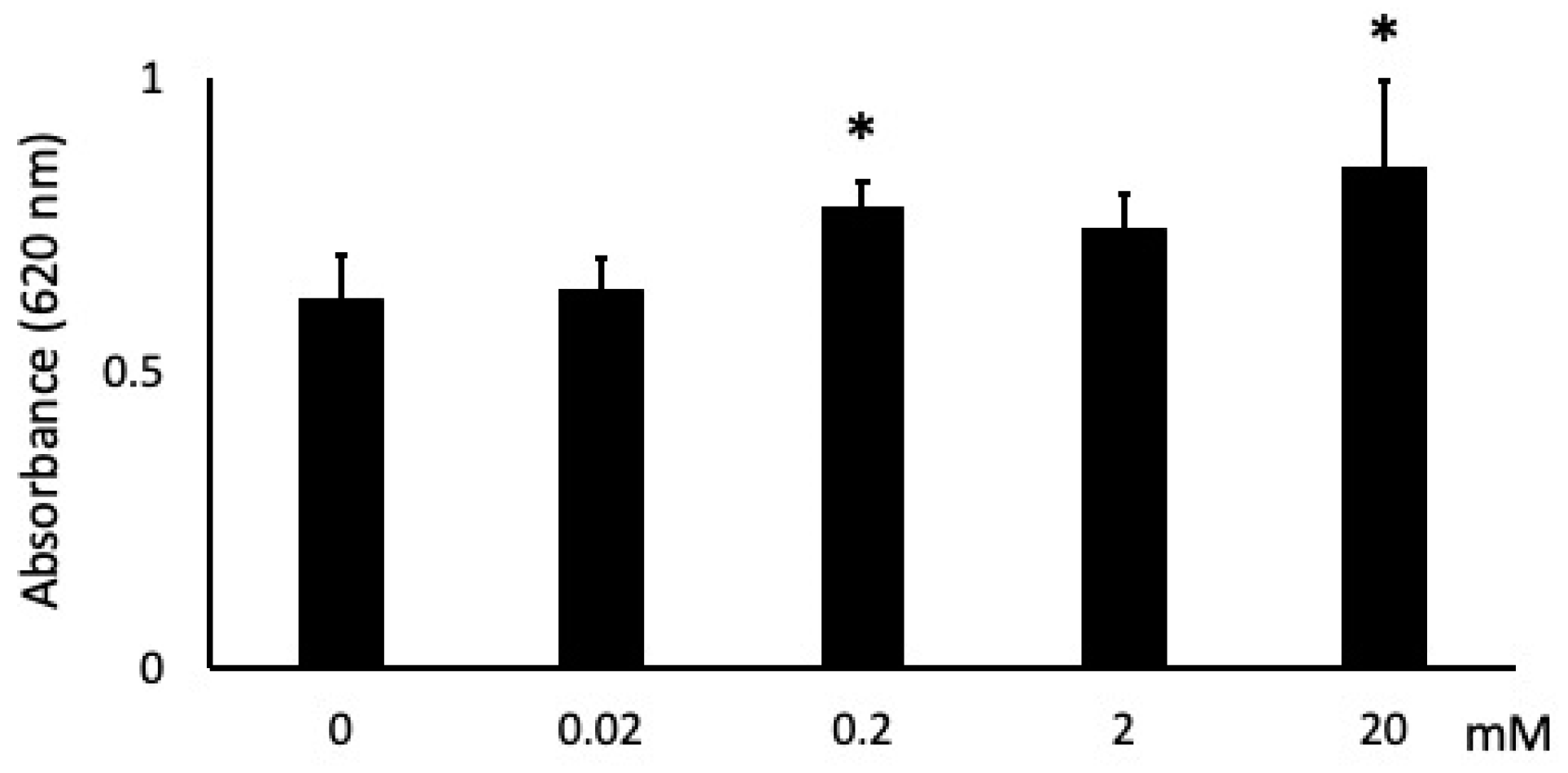
3.1. Butyric Acid Has Concentration-Dependent Positive and Negative Effects on Cell Proliferation
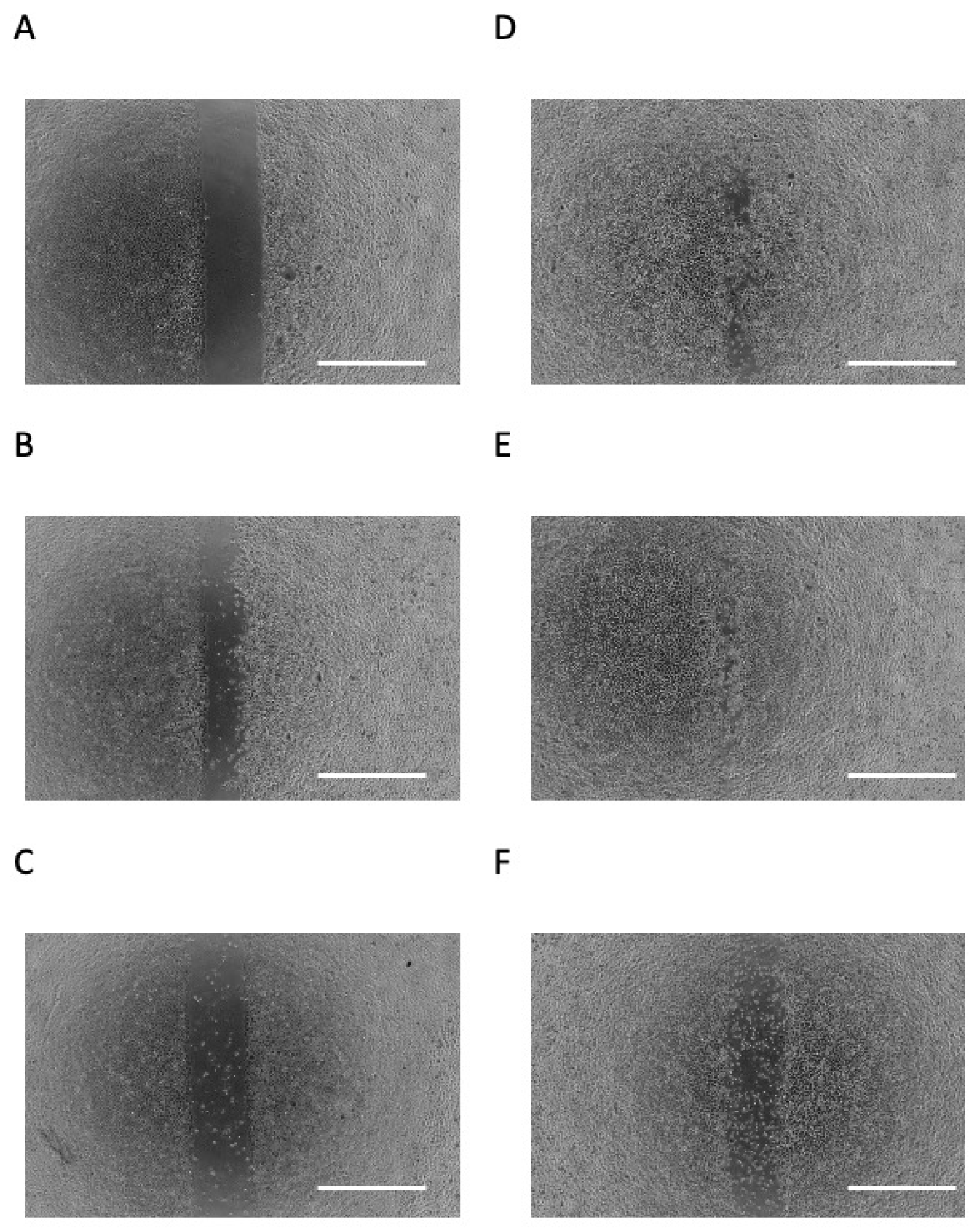
3.2. Butyric Acid Upregulates Cell Migration in a Concentration-Dependent Manner
3.3. Butyric Acid Enhances Cell Attachment
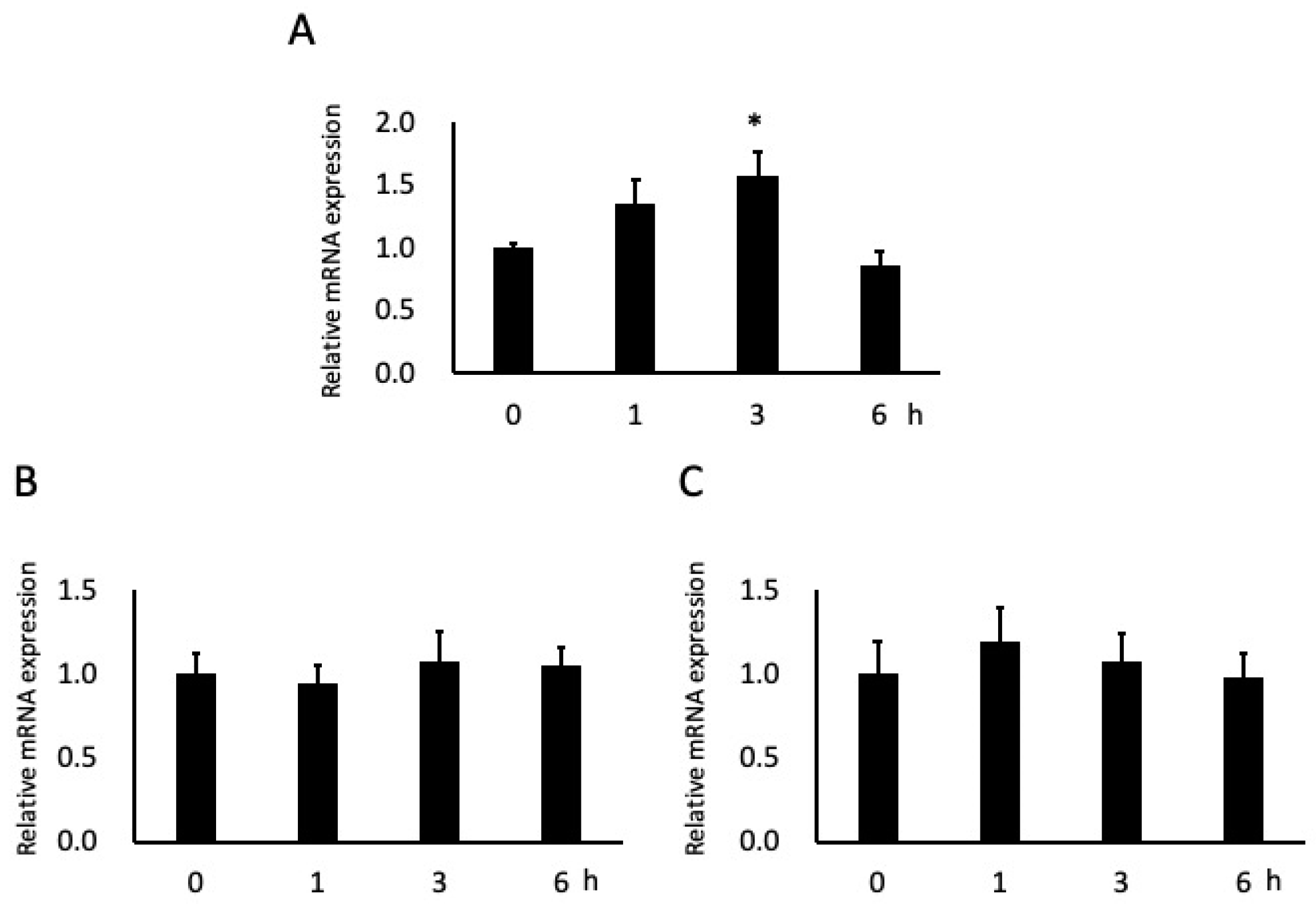
3.4. Effects of Butyric Acid on Adhesion Molecule Expression
3.5. CAGE and GO Term Enrichment Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S.; Aass, A.M.; Aimetti, M.; et al. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 4–60. [Google Scholar] [CrossRef]
- Arimatsu, K.; Yamada, H.; Miyazawa, H.; Minagawa, T.; Nakajima, M.; Ryder, M.I.; Gotoh, K.; Motooka, D.; Nakamura, S.; Iida, T.; et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci. Rep. 2014, 4, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossi, S.G.; Skrepcinski, F.B.; DeCaro, T.; Robertson, D.C.; Ho, A.W.; Dunford, R.G.; Genco, R.J. Treatment of Periodontal Disease in Diabetics Reduces Glycated Hemoglobin. J. Periodontol. 1997, 68, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Saremi, A.; Nelson, R.G.; Tulloch-Reid, M.; Hanson, R.L.; Sievers, M.L.; Taylor, G.W.; Shlossman, M.; Bennett, P.H.; Genco, R.; Knowler, W.C. Periodontal Disease and Mortality in Type 2 Diabetes. Diabetes Care 2004, 28, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Leng, W.-D.; Zeng, X.-T.; Kwong, J.S.; Hua, X.-P. Periodontal disease and risk of coronary heart disease: An updated meta-analysis of prospective cohort studies. Int. J. Cardiol. 2015, 201, 469–472. [Google Scholar] [CrossRef]
- e Silva Filho, W.S.; Casarin, R.C.; Junior, E.L.N.; Passos, H.M.; Sallum, A.W.; Gonçalves, R.B. Microbial diversity similarities in periodontal pockets and atheromatous plaques of cardiovascular disease patients. PLoS ONE 2014, 9, e109761. [Google Scholar] [CrossRef]
- Ohki, T.; Itabashi, Y.; Kohno, T.; Yoshizawa, A.; Nishikubo, S.; Watanabe, S.; Yamane, G.; Ishihara, K. Detection of periodontal bacteria in thrombi of patients with acute myocardial infarction by polymerase chain reaction. Am. Heart J. 2012, 163, 164–167. [Google Scholar] [CrossRef]
- Chou, Y.Y.; Lai, K.L.; Chen, D.Y.; Lin, C.H.; Chen, H.H. Rheumatoid arthritis risk associated with periodontitis exposure: A nationwide, population-based cohort study. PLoS ONE 2015, 10, e0139693. [Google Scholar] [CrossRef] [PubMed]
- Ziebolz, D.; Pabel, S.O.; Lange, K.; Krohn-Grimberghe, B.; Hornecker, E.; Mausberg, R.F. Clinical periodontal and microbiologic parameters in patients with rheumatoid arthritis. J. Periodontol. 2011, 82, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, P.; Karthikeyan, R.; Muthukumaraswamy, A.; Anand, J. A potential role of periodontal inflammation in Alzheimer’s disease. Oral Health Prev. Dent. 2017, 15, 7–12. [Google Scholar]
- Harding, A.; Gonder, U.; Robinson, S.J.; Crean, S.; Singhrao, S.K. Exploring the Association between Alzheimer’s Disease, Oral Health, Microbial Endocrinology and Nutrition. Front. Aging Neurosci. 2017, 9, 398. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.B.; Saito, M.T.; Matheus, F.C.; Prediger, R.D.; Yamada, E.S.; Maia, C.S.F.; Lima, R.R. Periodontitis and Alzheimer’s Disease: A Possible Comorbidity between Oral Chronic Inflammatory Condition and Neuroinflammation. Front. Aging Neurosci. 2017, 9, 327. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Liu, Y.; Meyer, M.; Giovannucci, E.; Joshipura, K. Periodontal disease, tooth loss, and cancer risk in male health professionals: A prospective cohort study. Lancet Oncol. 2008, 9, 550–558. [Google Scholar] [CrossRef] [Green Version]
- Tezal, M.; Sullivan, M.A.; Hyland, A.; Marshall, J.R.; Stoler, D.; Reid, M.E.; Loree, T.R.; Rigual, N.R.; Merzianu, M.; Hauck, L.; et al. Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer Epidemiol. Prev. Biomark. 2009, 18, 2406–2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, S.; Ren, H.; Guo, H.; Xing, W.; Liu, C.; Ji, Y.; Jiang, H.; Zhang, P.; Du, M. Periodontal infection with Porphyromonas gingivalis induces preterm birth and lower birth weight in rats. Mol. Oral Microbiol. 2018, 33, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, S.; Katagiri, S.; Maekawa, S.; Takeuchi, Y.; Komazaki, R.; Ohtsu, A.; Sasaki, N.; Shiba, T.; Watanabe, K.; Ishihara, K.; et al. Effect of Porphyromonas gingivalis infection in the placenta and umbilical cord in pregnant mice with low birth weight. Acta Odontol. Scand. 2018, 76, 433–441. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Korostoff, J.M. Revisiting the Page & Schroeder model: The good, the bad, and the unknown in periodontal host response forty years later. Periodontol. 2000 2017, 75, 116–151. [Google Scholar]
- Page, R.C.; Schroeder, H.E. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab. Investig. 1976, 34, 235. [Google Scholar] [PubMed]
- Pöllänen, M.T.; Salonen, J.I.; Uitto, V.-J. Structure and function of the tooth-epithelial interface in health and disease. Periodontol. 2000 2003, 31, 12–31. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, H.; Ochiai, K.; Suzuki, N.; Otsuka, K. Butyrate, a bacterial metabolite, induces apoptosis and autophagic cell death in gingival epithelial cells. J. Periodontal Res. 2010, 45, 626–634. [Google Scholar] [CrossRef]
- Niederman, R.; Buyle-Bodin, Y.; Lu, B.-Y.; Robinson, P.; Naleway, C. Short-chain Carboxylic Acid Concentration in Human Gingival Crevicular Fluid. J. Dent. Res. 1997, 76, 575–579. [Google Scholar] [CrossRef]
- Kurita-Ochiai, T.; Fukushima, K.; Ochiai, K. Volatile Fatty Acids, Metabolic By-products of Periodontopathic Bacteria, Inhibit Lymphocyte Proliferation and Cytokine Production. J. Dent. Res. 1995, 74, 1367–1373. [Google Scholar] [CrossRef]
- Kurita-Ochiai, T.; Ochiai, K.; Fukushima, K. Volatile Fatty Acid, Metabolic By-Product of Periodontopathic Bacteria, Induces Apoptosis in WEHI 231 and RAJI B Lymphoma Cells and Splenic B Cells. Infect. Immun. 1998, 66, 2587–2594. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, T.; Terashima, J.; Sasaki, D.; Shimoyama, Y.; Yaegashi, T.; Sasaki, M. Establishment and use of a three-dimensional ameloblastoma culture model to study the effects of butyric acid on the transcription of growth factors and laminin β3. Arch. Oral Biol. 2020, 118, 104845. [Google Scholar] [CrossRef]
- Ishikawa, T.; Terashima, J.; Shimoyama, Y.; Ohashi, Y.; Mikami, T.; Takeda, Y.; Sasaki, M. Effects of butyric acid, a bacterial metabolite, on the migration of ameloblastoma mediated by laminin 332. J. Oral Sci. 2020, 62, 435–438. [Google Scholar] [CrossRef]
- Schwarz, A.; Bruhs, A.; Schwarz, T. The Short-Chain Fatty Acid Sodium Butyrate Functions as a Regulator of the Skin Immune System. J. Investig. Dermatol. 2017, 137, 855–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, D.; Pan, Q.; Liu, X.L.; Yang, R.X.; Chen, Y.W.; Liu, C.; Fan, G.J. Clostridium butyricum B1 alleviates high-fat diet-induced steatohepatitis in mice via enterohepatic immunoregulation. J. Gastroenterol. Hepatol. 2017, 32, 1640–1648. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2014, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Shirasugi, M.; Nakagawa, M.; Nishioka, K.; Yamamoto, T.; Nakaya, T.; Kanamura, N. Relationship between periodontal disease and butyric acid produced by periodontopathic bacteria. Inflamm. Regen. 2018, 38, 23. [Google Scholar] [CrossRef]
- Takigawa, S.; Sugano, N.; Nishihara, R.; Koshi, R.; Murai, M.; Yoshinuma, N.; Ochiai, K.; Ito, K. The effect of butyric acid on adhesion molecule expression by human gingival epithelial cells. J. Periodontal Res. 2008, 43, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Hirose, K.; Isogai, E.; Mizugai, H.; Ueda, I. Adhesion of Porphyromonas gingivalis fimbriae to human gingival cell line Ca9-22. Oral Microbiol. Immunol. 1996, 11, 402–406. [Google Scholar] [CrossRef]
- Watanabe, K.; Yamaji, Y.; Umemoto, T. Correlation between cell-adherent activity and surface structure in Porphyromonas gingivalis. Oral Microbiol. Immunol. 1992, 7, 357–363. [Google Scholar] [CrossRef]
- Sakakibara, J.; Nagano, K.; Murakami, Y.; Higuchi, N.; Nakamura, H.; Shimozato, K.; Yoshimura, F. Loss of adherence ability to human gingival epithelial cells in S-layer protein-deficient mutants of Tannerella forsythensis. Microbiology 2007, 153, 866–876. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, H.E.; Listgarten, M.A. The Junctional Epithelium: From Strength to Defense. J. Dent. Res. 2003, 82, 158–161. [Google Scholar] [CrossRef]
- Yajima-Himuro, S.; Oshima, M.; Yamamoto, G.; Ogawa, M.; Furuya, M.; Tanaka, J.; Nishii, K.; Mishima, K.; Tachikawa, T.; Tsuji, T.; et al. The junctional epithelium originates from the odontogenic epithelium of an erupted tooth. Sci. Rep. 2015, 4, 4867. [Google Scholar] [CrossRef]
- Seki, T.; Aizawa, R.; Tanaka, J.; Yajima-Himuro, S.; Kato, M.; Tanaka, K.; Mishima, K.; Yamamoto, M. Establishment of mouse gingival junctional epithelial cell line using a bioengineered tooth system. Biochem. Biophys. Res. Commun. 2018, 497, 167–172. [Google Scholar] [CrossRef]
- Hu, Y.; Rao, S.S.; Wang, Z.X.; Cao, J.; Tan, Y.J.; Luo, J.; Li, H.M.; Zhang, W.S.; Chen, C.Y.; Xie, H. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 2018, 8, 169–184. [Google Scholar] [CrossRef]
- Nguyen, P.A.; Pham, T.A.V. Effects of platelet-rich plasma on human gingival fibroblast proliferation and migration in vitro. J Appl. Oral Sci. 2018, 26, e20180077. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, T.; Wondimu, Z.; Oikawa, Y.; Ingerpuu, S.; Virtanen, I.; Patarroyo, M. Monoclonal antibodies to human laminin α4 chain globular domain inhibit tumor cell adhesion and migration on laminins 411 and 421, and binding of α6β1 integrin and MCAM to α4-laminins. Matrix Biol. 2014, 36, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Wondimu, Z.; Oikawa, Y.; Gentilcore, G.; Kiessling, R.; Egyhazi Brage, S.; Hansson, J.; Patarroyo, M. Laminins 411 and 421 differentially promote tumor cell migration via α6β1 integrin and MCAM (CD146). Matrix Biol. 2014, 38, 69–83. [Google Scholar] [CrossRef]
- Goto, Y.; Ibi, M.; Sato, H.; Tanaka, J.; Yasuhara, R.; Aota, K.; Azuma, M.; Fukada, T.; Mishima, K.; Irié, T. PLAG1 enhances the stemness profiles of acinar cells in normal human salivary glands in a cell type-specific manner. J. Oral Biosci. 2020, 62, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Haberle, V.; Forrest, A.R.; Hayashizaki, Y.; Carninci, P.; Lenhard, B. CAGEr: Precise TSS data retrieval and high-resolution promoterome mining for integrative analyses. Nucleic Acids Res. 2015, 43, e51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frith, M.C.; Valen, E.; Krogh, A.; Hayashizaki, Y.; Carninci, P.; Sandelin, A. A code for transcription initiation in mammalian genomes. Genome Res. 2008, 18, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Blottière, H.M.; Buecher, B.; Galmiche, J.P.; Cherbut, C. Molecular analysis of the effect of short-chain fatty acids on intestinal cell proliferation. Proc. Nutr. Soc. 2003, 62, 101–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siavoshian, S.; Blottiere, H.M.; Cherbut, C.; Galmiche, J.P. Butyrate stimulates cyclin D and p21 and inhibits cyclin-dependent kinase 2 expression in HT-29 colonic epithelial cells. Biochem. Biophys. Res Commun. 1997, 232, 169–172. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Kikuchi, K.; González-Alva, P.; Inoue, H.; Noguchi, Y.; Tsuchiya, H.; Hayashi, J.; Shin, K.; Ochiai, K.; Kaoru, K. Association of butyric acid produced by periodontopathic bacteria with progression of oral cancer. J. Cancer Sci. Ther. 2010, 2, 026–032. [Google Scholar]
- Durbeej, M. Laminins. Cell Tissue Res. 2010, 339, 259–268. [Google Scholar] [CrossRef]
- Anbarasan, C.; Bavanilatha, M.; Latchumanadhas, K.; Ajit Mullasari, S. ICAM-1 molecular mechanism and genome wide SNP’s association studies. Indian Heart J. 2015, 67, 282–287. [Google Scholar] [CrossRef] [Green Version]
- Yuille, S.; Reichardt, N.; Panda, S.; Dunbar, H.; Mulder, I.E. Human gut bacteria as potent class I histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid. PLoS ONE 2018, 13, e0201073. [Google Scholar] [CrossRef] [Green Version]

| Category | Term | Count | Gene ID | p-Value |
|---|---|---|---|---|
| GOTERM_BP_DIRECT | Immune response | 3 | Ccl5, Il7, Tnfsf13 | 4.60 × 10−2 |
| GOTERM_BP_DIRECT | Positive regulation of phosphatidylinostiol 3-kinase signaling | 2 | Ccl5, Tgfb2 | 7.80 × 10−2 |
| GOTERM_BP_DIRECT | Neutrophil chemotaxis | 2 | Ccl5, Epgn | 8.40 × 10−2 |
| GOTERM_BP_DIRECT | MAPK cascade | 2 | Ccl5, Tgfb2 | 8.40 × 10−2 |
| GOTERM_BP_DIRECT | Positive regulation of epitherial cell proliferation | 2 | Ccl5, Epgn | 9.20 × 10−2 |
| GOTERM_CC_DIRECT | Extracellular space | 7 | Actg1, Aga, Ccl5, Epgn, Il7, Tgfb2, Tnfsf13 | 9.90 × 10−3 |
| GOTERM_MF_DIRECT | Cytokine activity | 4 | Ccl5, Il7, Tgfb2, Tnfsf13 | 2.10 × 10−3 |
| GOTERM_MF_DIRECT | Growth factor activity | 3 | Epgn, Il7, Tgfb2 | 1.30 × 10−2 |
| GOTERM_MF_DIRECT | Protein self-association | 2 | Aga, Ccl5 | 7.00 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishikawa, T.; Sasaki, D.; Aizawa, R.; Shimoyama, Y.; Yamamoto, M.; Irié, T.; Sasaki, M. Effect of Butyric Acid in the Proliferation and Migration of Junctional Epithelium in the Progression of Periodontitis: An In Vitro Study. Dent. J. 2021, 9, 44. https://0-doi-org.brum.beds.ac.uk/10.3390/dj9040044
Ishikawa T, Sasaki D, Aizawa R, Shimoyama Y, Yamamoto M, Irié T, Sasaki M. Effect of Butyric Acid in the Proliferation and Migration of Junctional Epithelium in the Progression of Periodontitis: An In Vitro Study. Dentistry Journal. 2021; 9(4):44. https://0-doi-org.brum.beds.ac.uk/10.3390/dj9040044
Chicago/Turabian StyleIshikawa, Taichi, Daisuke Sasaki, Ryo Aizawa, Yu Shimoyama, Matsuo Yamamoto, Tarou Irié, and Minoru Sasaki. 2021. "Effect of Butyric Acid in the Proliferation and Migration of Junctional Epithelium in the Progression of Periodontitis: An In Vitro Study" Dentistry Journal 9, no. 4: 44. https://0-doi-org.brum.beds.ac.uk/10.3390/dj9040044






