The Impact of Sport Training on Oral Health in Athletes
Abstract
:1. Introduction
2. The Oral Ecosystem and the Saliva Diagnostic Function
3. Sports and Oral Health
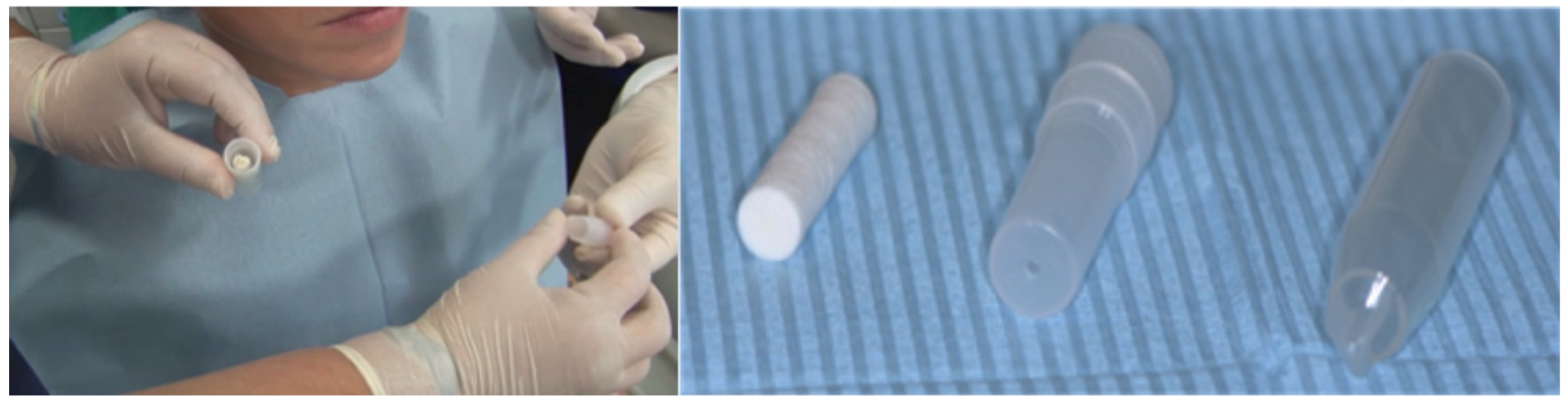
4. Sport Mouthguard: Effects on the Oral Ecosystem
5. Sport Mouthguard in the Prevention of Oral Diseases
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gallagher, J.; Ashley, P.; Needlman, I. Implementation of a behavioural change intervention to enhance oral health behaviours in elite athletes: A feasibility study. BMJ Open Sport Exerc. Med. 2020. [Google Scholar] [CrossRef]
- D’Ercole, S.; Tieri, M.; Martinelli, D.; Tripodi, D. The effect of swimming on oral health status: Competitive versus non-competitive athletes. J. Appl. Oral Sci. 2016, 24, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Spinas, E.; Mameli, A.; Giannetti, L. Traumatic dental injuries resulting from sports activities; immediate treatment and five years follow-up: An observational study. Open Dent. J. 2018, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Giuca, M.R.; Pasini, M.; Tecco, S. Levels of salivary immunoglobulins and periodontal evaluation in smoking patients. BMC Immunol. 2014, 15. [Google Scholar] [CrossRef] [Green Version]
- Nieman, D.C.; Henson, D.A.; Fagoaga, O.R. Change in salivary IgA following a competitive marathon race. Int. J. Sports Med. 2002, 69–75. [Google Scholar] [CrossRef]
- Knapik, J.J.; Marshall, S.W.; Lee, R.B.; Darakjy, S.S.; Jones, S.B.; Mitchener, T.A.; Dela Cruz, G.; Jones, B.H. Mouthguards in sport activities: History, physical properties and injury prevention effectiveness. Sports Med. 2007, 37, 117–144. [Google Scholar] [CrossRef]
- Samaranayake, L.; Matsubara, V.H. Normal oral flora and the oral ecosystem. Dent. Clin. N. Am. 2017, 61, 199–215. [Google Scholar] [CrossRef]
- Marsh, P.D. In sickness and in health-what does the oral microbiome mean to us? An ecological perspective. Adv. Dent. Res. 2018, 29, 60–65. [Google Scholar] [CrossRef]
- Marcotte, H.; Lavoie, M.C. Oral microbial ecology and the role of salivary immunoglobulin A. Microbiol. Mol. Biol. Rev. 1998, 62, 71–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufman, E.; Lamster, I.B. The diagnostic applications of saliva—A review. Crit. Rev. Oral Biol. Med. 2002, 13, 197–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amerongen, A.V.; Veerman, E.C. Saliva--the defender of the oral cavity. Oral Dis. 2002, 8, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Mese, H.; Matsuo, R.J. Salivary secretion, taste and hyposalivation. Oral Rehabil. 2007, 34, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Pfaffe, T.; Cooper-White, J.; Beyerlei, P.; Kostner, K.; Punyadeera, C. Diagnostic potential of saliva: Current state and future applictions. Clin. Chem. 2012, 36, 126–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Godoy, F.; Hicks, M.J. Maintaining the integrity of the enamel surface: The role of dental biofilm, saliva and preventive agents in enamel demineralization and remineralization. J. Am. Dent. Assoc. 2008, 139, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Segal, A.; Wong, D.T. Salivary diagnostics: Enhancing disease detection and making medicine better. Eur. J. Dent. Educ. 2008, 12, 22–29. [Google Scholar] [CrossRef]
- Wong, D.T. Salivary diagnostics: Scientific and clinical frontiers. Adv. Dent. Res. 2011, 23, 350–352. [Google Scholar] [CrossRef]
- Ilea, A.; Andrei, V.; Feurdean, C.N.; Băbțan, A.M.; Petrescu, N.B.; Câmpian, R.S.; Boșca, A.B.; Ciui, B.; Tertiș, M.; Săndulescu, R.; et al. Saliva, a magic biofluid available for multilevel assessment and a mirror of general health-a systematic review. Biosensors (Basel) 2019, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Mandel, I.D. Salivary diagnosis: More than a lick and a promise. J. Am. Dent. Assoc. 1993, 124, 85–87. [Google Scholar] [CrossRef]
- Papacosta, E.; Nassis, G.P. Saliva as a tool for monitoring steroid, peptide and immune markers in sport and exercise science. J. Sci. Med. Sport 2011, 5, 424–434. [Google Scholar] [CrossRef]
- Wang, A.; Wang, C.; Tu Wong, D.T. Oral biofluid biomarker research: Current status and emerging frontiers. Diagnostics (Basel) 2016, 6, 45. [Google Scholar] [CrossRef]
- Ushiki, K.; Tsunekawa, K.; Shoho, Y.; Martha, L.; Ishigaki, H.; Matsumoto, R.; Yanagawa, Y.; Nakazawa, A.; Yoshida, A.; Nakajima, K.; et al. Assessment of exercise-induced stress by automated measurement of salivary cortisol concentrations within the circadian rhythm in Japanese female long-distance runners. Sports Med. Open 2020, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.E.; Jäger, R.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Townsend, J.R.; West, N.P.; Black, K.; Gleeson, M.; Pyne, D.B.; et al. The athletic gut microbiota. J. Int. Soc. Sports Nutr. 2020, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Finamore, A.; Peluso, I.; Cauli, O. Salivary stress/immunological markers in crohn’s disease and ulcerative colitis. Int. J. Mol. Sci. 2020, 13, 8562. [Google Scholar] [CrossRef]
- Hegde, M.N.; Attavar, S.H.; Shetty, N.; Hegde, N.D.; Hegde, N.N. Saliva as a biomarker for dental caries: A systematic review. J. Conserv. Dent. 2019, 22, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Mummolo, S.; Marchetti, E.; Giuca, M.R. In-office bacteria test for a microbial monitoring during the conventional and self-ligating orthodontic treatment. Head Face Med. 2013, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubala, E.; Strzelecka, P.; Grzegocka, M.; Lietz-Kijak, D.; Gronwald, H.; Piotr, S.; Kijak, E. A review of selected studies that determine the physical and chemical properties of saliva in the field of dental treatment. Biomed Res. Int. 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Hemadi, A.S.; Huang, R.; Zhou, Y.; Zou, J. Salivary proteins and microbiota as biomarkers for early childhood caries risk assessment. Int. J. Oral Sci. 2017, 9, e1. [Google Scholar] [CrossRef] [Green Version]
- Bel’skaya, L.V.; Kosenok, V.K.; Sarf, E.A. Chronophysiological features of the normal mineral composition of human saliva. Arch. Oral Biol. 2017, 82, 286–292. [Google Scholar] [CrossRef]
- Bel’skaya, L.V.; Sarf, E.A.; Solomatin, D.V. Age and gender characteristics of the infrared spectra of normal human saliva. Appl. Spectrosc. 2020, 74, 536–543. [Google Scholar] [CrossRef]
- Mummolo, S.; Tieri, M.; Tecco, S. Clinical evaluation of salivary indices and levels of Streptococcus mutans and Lactobacillus in patients treated with Occlus-o-Guide. Eur. J. Paediatr. Dent. 2014, 15, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Needleman, I. Oral health and elite sport performance. Br. J. Sports Med. 2015, 49, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Escartin, J.L.; Arnedo, A.; Pinto, V.; Vela, M.J. A study of dental staining among competitive swimmers. Community Dent. Oral Epidemiol. 2000, 28, 10–17. [Google Scholar] [CrossRef]
- Ashley, P.; Di Iorio, A.; Cole, E. Oral health of elite athletes and association with performance: A systematic review. Br. J. Sports Med. 2015, 49, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.E.; Bourgeois, D.; Ogawa, H.; Estupinan-Day, S.; Ndiaye, C. The global burden of oral diseases and risks to oral health. Bull. World Health Organ. 2005, 83, 661–669. [Google Scholar] [PubMed]
- Spinas, E.; Giannetti, L.; Mameli, A.; Re, D. Dental injuries in young athletes, a five-year follow-up study. Eur. J. Paediatr. Dent. 2018, 19, 187–193. [Google Scholar]
- Bourgeois, D.; Inquimbert, C.; Ottolenghi, L.; Carrouell, F. Periodontal pathogens as risk factors of cardiovascular diseases, diabetes, rheumatoid arthritis, cancer, and chronic obstructive pulmonary disease-is there cause for consideration? Microorganisms 2019, 7, 424. [Google Scholar] [CrossRef] [Green Version]
- Gay-Escoda, C.; Vieira-Duarte-Pereira, D.M.; Ardèvol, J.; Pruna, R.; Fernandez, J.; Valmaseda-Castellòn, E. Study of the effect of oral health on physical condition of professional soccer players of the Football Club Barcelona. Med. Oral Patol. Oral Cir. Bucal 2011, 16, 436–439. [Google Scholar] [CrossRef] [Green Version]
- D’Ercole, S.; Tripodi, D.; Ristoldo, F.; Quaranta, F.; Amaddeo, P. Analysis of oral health status and of salivary factors in young soccer players: A pilot study. Med. Dello Sport 2013, 66, 71–80. [Google Scholar]
- D’Ercole, S.; Tripodi, D. The effect of swimming on oral ecological factors. J. Biol. Regul. Homeost. Agents 2013, 2, 551–558. [Google Scholar]
- Tanabe-Ikegawa, M.; Takahashi, T.; Churei, H.; Mitsuyama, A.; Ueno, T. Interactive effect of rehydration with diluted sports drink and water gargling on salivary flow, pH, and buffering capacity during ergometer exercise in young adult volunteers. J. Oral Sci. 2018, 60, 269–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bretz, W.A.; Carrilho, M.R. Salivary parameters of competitive swimmers at gas-chlorinated swimming-pools. J. Sports Sci. Med. 2013, 12, 207–208. [Google Scholar]
- Lussi, A.; Schlueter, N.; Rakhmatullina, E.; Ganss, C. Dental erosion: An overview with emphasis on chemical and histopathological aspects. Caries Res. 2011. [Google Scholar] [CrossRef]
- Anttonen, V.; Kemppainen, A.; Niinimaa, A.; Pesonen, P.; Tjaderhane, L.; Jaana, L. Dietary and oral hygiene habits of active athletes and adolescents attending ordinary junior high schools. Int. J. Paediatr. Dent. 2014, 24, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Committee on Nutrition and the Council on Sport Medicine and Fitness. Sports drinks and energy drinks for children and adolescents: Are they appropriate? Pediatrics 2011, 127, 1182–1189. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarek, W. The status of mineralized dental tissues in young competitive swimmers. Acad. Med. Stetin. 2010, 56, 81–86. [Google Scholar]
- Grippo, J.O.; Simring, M.; Coleman, T.A. Abfraction, abrasion, biocorrosion, and the enigma of noncarious cervical lesions: A 20-year perspective. J. Esthet. Restor. Dent. 2012, 24, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zou, Y.; Ding, G. Correction: Dietary factors associated with dental erosion: A meta-analysis. PLoS ONE 2016, 11, e0161518. [Google Scholar] [CrossRef]
- Chrysanthakopoulos, N.A. Prevalence of tooth erosion and associated factors in 13-16-year old adolescents in Greece. J. Clin. Exp. Dent. 2012, 4, 160–166. [Google Scholar] [CrossRef]
- Sheiham, A.; James, W.P. Diet and dental caries: The pivotal role of free sugars reemphasized. J. Dent. Res. 2015, 94, 1341–1347. [Google Scholar] [CrossRef]
- Bellomo, R.G.; Tripodi, D.; Bosna, C.; D’Ercole, S.; Barassi, G.; Porreca, A.; Veraldi, R.; Prosperi, L.; Barbato, C. Mediterranean diet and physical activity improve posture, fat mass and salivary pH. J. Biol. Regul. Homeost. Agents 2018, 32, 1317–1321. [Google Scholar] [PubMed]
- Kaur, G.; Mangat, S.S. Attitude toward mouthguard utilization among North Indian school children. J. Int. Soc. Prev. Community Dent. 2016, 6, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Spinas, E.; Aresu, M.; Giannetti, L. Use of mouth guard in basketball: Observational study of a group of teenagers with and without motivational reinforcement. Eur. J. Paediatr. Dent. 2014, 15, 392–396. [Google Scholar]
- D’Ercole, S.; Martinelli, D.; Tripodi, D. Influence of sport mouthguard on the ecological factor of the children oral cavity. BMC Oral Health 2014, 14, 97. [Google Scholar] [CrossRef] [Green Version]
- Chiavaroli, A.; D’Ercole, S.; Fulco, D.; Ferrante, C.; Orlando, G.; Recinella, L.; Tripodi, D. The use of customized mouthguards during the training produced protective effects on salivary factors of young athletes. EJPD 2021, (in press). [Google Scholar]
- Schultz Martins, R.; Girouard, P.; Elliott, E.; Mekary, S. Physiological responses of a jaw-repositioning custom-made mouthguard on airway and their effects on athletic performance. J. Strength Cond. Res. 2018. [Google Scholar] [CrossRef]
- Spicuzza, L.; Parisi, G.F.; Tardino, L.; Ciancio, N.; Nenna, R.; Midulla, F.; Leonardi, S. Exhaled markers of antioxidant activity and oxidative stress in stable cystic fibrosis patients with moderate lung disease. J. Breath Res. 2018, 12, 026010. [Google Scholar] [CrossRef] [PubMed]
- Glass, R.T.; Conrad, R.S.; Wood, C.R. Protective athletic mouthguards: Do they cause harm? Sports Health 2009, 1, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Glass, R.T.; Wood, C.R.; Bullard, J.W.; Conrad, R.S. Possible disease transmission by contaminated mouthguards in two young football players. Gen. Dent. 2007, 55, 436–440. [Google Scholar] [PubMed]
- Glass, R.T.; Conrad, R.S.; Kohler, G.A.; Warren, A.J.; Bullard, J.W. Microbiota found in protective athletic mouthguards. Sports Health 2011, 3, 244–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glass, R.T.; Bullard, J.W.; Conrad, R.S. The contamination of protective mouthguards: A characterization of the microbiota found in football players’ protective mouthguards as compared to the oral microbiota found in first-year medical students. J. Amer. Dent. Inst. Cont. Educ. 2006, 93, 23–38. [Google Scholar]
- Batoni, G.; Pardini, M.; Giannotti, A.; Ota, F.; Giuca, M.R.; Gabriele, M.; Campa, M.; Senesi, S. Effect of removable orthodontic appliances on oral colonisation by mutans streptococci in children. Eur. J. Oral Sci. 2001, 109, 388–392. [Google Scholar] [CrossRef]
- Sanpei, S.; Endo, T.; Shimooka, S. Caries risk factors in children under treatment with sectional brackets. Angle Orthod. 2010, 80, 509–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lara-Carrillo, E.; Montiel-Bastida, N.M.; Sanchez-Perez, L.; Alais-Tavira, J. Effect of orthodontic treatment on saliva, plaque and the levels of Streptococcus mutans and Lactobacillus. Med. Oral Patol. Oral Cir. Bucal 2010, 15, 924–929. [Google Scholar] [CrossRef] [Green Version]
- Raquel, G.; Namba, E.L.; Bonotto, D.; Ribeiro Rosa, E.A.; Trevilatto, P.C.; Naval Machado, M.Â.; Vianna-Lara, M.S.; Azevedo-Alanis, L.R. The use of a custom-made mouthguard stabilizes the electromyographic activity of the masticatory muscles among Karate-Dō athletes. J. Bodyw. Mov. Ther. 2016, 21, 109–116. [Google Scholar] [CrossRef]
- Mummolo, S.; D’Ercole, S.; Marchetti, E.; Campanella, V.; Martinelli, D.; Marzo, G.; Tripodi, D. Oral antiseptic and periodontitis: A clinical and microbiological study. Oral Health Dent. Manag. 2014, 13, 698–702. [Google Scholar] [PubMed]
- D’Ercole, S.; Tieri, M.; Fulco, D.; Martinelli, D.; Tripodi, D. The use of chlorhexidine in mouthguards. J. Biol. Regul. Homeost. Agents 2017, 31, 487–493. [Google Scholar] [PubMed]
- D’Ercole, S.; Tieri, M.; Martinelli, D.; Ciaravino, C.; Fulco, D.; Tripodi, D. Microbial contamination and disinfection of sport mouthguard: In vitro study. Curr. Microbiol. 2020, 77, 246–253. [Google Scholar] [CrossRef]
- Tripodi, D.; Martinelli, D.; Ciaravino, C.; Fulco, D.; Tieri, M.; D’Ercole, S. The use of casein in sport mouthguards: Microbiological and ecological variations in oral cavity. J. Biol. Regul. Homeost. Agents 2018, 32, 1045–1049. [Google Scholar]
- Hegde, R.J.; Thakkar, J.B. Comparative evaluation of the effects of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) and xylitol-containing chewing gum on salivary flow rate, pH and buffering capacity in children: An in vivo study. J. Indian Soc. Pedod. Prev. Dent. 2017, 35, 332–337. [Google Scholar] [CrossRef]
- Nagai, K.; Domon, H.; Oda, M.; Shirai, T.; Ohsumi, T.; Terao, Y.; Arai, Y. Antimicrobial activity of ethylene-vinyl acetate containing bioactive filler against oral bacteria. Dent. Mater. J. 2017, 36, 784–790. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, Y.; Churei, H.; Takeuchi, Y.; Wada, T.; Uo, M.; Izumi, Y.; Ueno, T. Novel antibacterial mouthguard material manufactured using silver-nanoparticle-embedded ethylene-vinyl acetate copolymer masterbatch. Dent. Mater. J. 2018, 37, 437–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namba, E.; Strapasson, A.; Jarzynski, J.; Baratto, S.P.; Tomazinho, P.H. Sanitization of sports mouthguards. RSBO 2013, 10, 72–75. [Google Scholar]
- Barton, L. Mouthguards: Daily Sanitizing between Uses Urged. 2016. Available online: https://www.momsteam.com/health-safety/mouth-guards-daily-sanitizing-between-uses-urged (accessed on 1 April 2021).
- Ogawa, T.; Yamasaki, S.; Honda, M.; Terao, Y.; Kawabata, S.; Maeda, Y. Long-term survival of salivary streptococci on dental devices made of ethylene vinyl acetate. Int. J. Oral Sci. 2012, 4, 14–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gould, T.E.; Piland, S.G.; Shin, J.; Hoyle, C.E.; Nazarenko, S. Characterization of mouthguard materials: Physical and mechanical properties of commercialized products. Dent. Mater. 2009, 25, 771–780. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripodi, D.; Cosi, A.; Fulco, D.; D’Ercole, S. The Impact of Sport Training on Oral Health in Athletes. Dent. J. 2021, 9, 51. https://0-doi-org.brum.beds.ac.uk/10.3390/dj9050051
Tripodi D, Cosi A, Fulco D, D’Ercole S. The Impact of Sport Training on Oral Health in Athletes. Dentistry Journal. 2021; 9(5):51. https://0-doi-org.brum.beds.ac.uk/10.3390/dj9050051
Chicago/Turabian StyleTripodi, Domenico, Alessia Cosi, Domenico Fulco, and Simonetta D’Ercole. 2021. "The Impact of Sport Training on Oral Health in Athletes" Dentistry Journal 9, no. 5: 51. https://0-doi-org.brum.beds.ac.uk/10.3390/dj9050051





