Autofluorescence Detection Method for Dental Plaque Bacteria Detection and Classification: Example of Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and Streptococcus mutans
Abstract
:1. Introduction
2. Materials and Methods
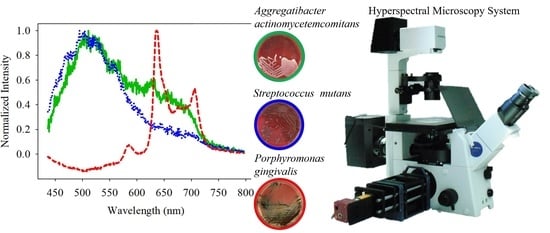
2.1. Hyperspectral Microscopy System
2.2. Specific Consideration for Eliminating the Excitation Light
2.3. Object Slide of Microscope Necessary for Autofluorescence Measurement
2.4. Culture the Bacteria
3. Results and Analysis
3.1. Photobleaching of PG
3.2. Classification of PG/AA/SM
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Health Promotion Administration, Ministry of Health and Welfare of Taiwan. Annual Report. Available online: https://health99.hpa.gov.tw/media/public/pdf/21617.pdf (accessed on 22 April 2021).
- Beveridge, T.J. Use of the Gram stain in microbiology. Biotech. Histochem. 2001, 76, 111–118. [Google Scholar] [CrossRef]
- Bartholomew, J.W.; Mittwer, T. The gram stain. In Bacteriological Reviews; University of Southern California: Los Angeles, CA, USA, 1952; Volume 16, pp. 1–29. [Google Scholar] [CrossRef]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas Gingivalis: An overview of periodontopathic pathogen below the gum line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H.; Patil, A.G.; Velusamy, S.K. Aggregatibacter Actinomycetemcomitans (AA) under the radar: Myths and misunderstandings of AA and its role in aggressive periodontitis. Front. Immunol. 2019, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- Natrajan, V.K.; Christensen, K.T. Encyclopedia of Microfluidics and Nanofluidics; Li, D., Ed.; Springer: Boston, MA, USA, 2008; ISBN 978-0-387-49000-7. [Google Scholar] [CrossRef]
- Coulthwaite, L.; Pretty, I.A.; Smith, P.W.; Higham, S.M.; Verran, J. The microbiological origin of fluorescence observed in plaque on dentures during QLF analysis. Caries Res. 2006, 40, 112–116. [Google Scholar] [CrossRef]
- Rechmann, P.; Liou, S.W.; Rechmann, B.M.; Featherstone, J.D. SOPROCARE-450 nm wavelength detection tool for microbial plaque and gingival inflammation: A clinical study. Lasers Dent. XX 2014, 8929, 892906. [Google Scholar]
- Joseph, B.; Prasanth, C.S.; Jayanthi, J.L.; Presanthila, J.; Subhash, N. Detection and quantification of dental plaque based on laser-induced autofluorescence intensity ratio values. J. Biomed. Opt. 2015, 20, 048001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Gomez, J.; Khan, S.; Peru, D.; Ellwood, R. Red fluorescence imaging for dental plaque detection and quantification: Pilot study. J. Biomed. Opt. 2017, 22, 096008. [Google Scholar] [CrossRef] [PubMed]
- Van der Veen, M.H.; Thomas, R.Z.; Huysmans, M.C.D.N.J.M.; de Soet, J.J. Red autofluorescence of dental plaque bacteria. Caries Res. 2006, 40, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.S.; Mohanty, S.; Jayanthi, J.L.; Varughese, J.M.; Balan, A.; Subhash, N. Clinical trial for detection of dental caries using laser-induced fluorescence ratio reference standard. J. Biomed. Opt. 2010, 15, 027001. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.S.; Kang, S.M.; Ko, H.Y.; Kwon, H.K.; Kim, B.I. Association between the cariogenicity of a dental microcosm biofilm and its red fluorescence detected by Quantitative Light-induced Fluorescence-Digital (QLF-D). J. Dent. 2013, 41, 1264–1270. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, H.; Xu, Y.; Lin, B.; Chen, H. Discrimination of dental caries using colorimetric characteristics of fluorescence spectrum. Caries Res. 2015, 49, 401–407. [Google Scholar] [CrossRef]
- Lee, E.S.; Yim, H.K.; Lee, H.S.; Choi, J.H.; Kwon, H.K.; Kim, B.I. Plaque autofluorescence as potential diagnostic targets for oral malodor. J. Biomed. Opt. 2016, 21, 085005. [Google Scholar] [CrossRef] [Green Version]
- Konigm, K.; Flelviming, G.; Hibst, R. Laser—Induced Autofluorescen Ce Spectroscopy of Dental Caries. Cell. Mol. Biol. 1998, 44, 1293–1300. [Google Scholar]
- Hsieh, Y.F.; Ou-Yang, M.; Duann, J.R.; Chiou, J.C.; Chang, N.W.; Jan, C.I.; Tsai, M.H.; Wu, S.D.; Lin, Y.J.; Lee, C.C. Develop-ment of a novel embedded relay lens microscopic hyperspectral imaging system for cancer diagnosis: Use of the mice with oral cancer to be the example. Int. J. Spectrosc. 2012, 2012, 710803. [Google Scholar] [CrossRef] [Green Version]
- Sharma, Y.K.; Singh, R.K.; Pal, S. Praseodymium ion doped sodium borosilicate glasses: Energy interaction and radiative properties. Am. J. Condens. Matter Phys. 2015, 5, 10–18. [Google Scholar] [CrossRef]
- Herman, B.; Parry-Hill, M.J.; Johnson, D.I.; Davidson, M.W. Photobleaching. Available online: http://micro.magnet.fsu.edu/primer/java/fluorescence/photobleaching/ (accessed on 4 April 2021).
- Hope, C.K.; de Josselin de Jong, E.; Field, M.R.T.; Valappil, S.P.; Higham, S.M. Photobleaching of red fluorescence in oral bio-films. J. Periodontal Res. 2011, 46, 228–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fyrestam, J. Porphyrins and Heme in Microorganisms: Porphyrin Content and Its Relation to Phototherapy and Antimicrobial Treatments In Vivo and In Vitro. Ph.D. Thesis, Stockholm University, Stockholm, Sweden, 19 January 2018. [Google Scholar]
- Quiroz-Segoviano, R.I.Y.; Serratos, I.N.; Rojas-González, F.; Tello-Solís, S.R.; Sosa-Fonseca, R.; Medina-Juárez, O.; Menchaca-Campos, C.; García-Sánchez, M.A. On Tuning the Fluorescence Emission of Porphyrin Free Bases Bonded to the Pore Walls of Organo-Modified Silica. Molecules 2014, 19, 2261–2285. [Google Scholar] [CrossRef] [Green Version]
- Uttamlal, M.; Holmes-Smith, A.S. The excitation wavelength dependent fluorescence of porphyrins. Chem. Phys. Lett. 2008, 454, 223–228. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Porphyrin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Porphyrin (accessed on 24 April 2021).
- National Center for Biotechnology Information. Tetraphenylporphyrin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tetraphenylporphyrin (accessed on 24 April 2021).
- National Center for Biotechnology Information. Protoporphyrin IX. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Protoporphyrin-IX (accessed on 24 April 2021).
- National Center for Biotechnology Information. Heme. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Heme (accessed on 24 April 2021).
- Volgenant, C.M.; van der Veen, M.H.; de Soet, J.J.; ten Cate, J.M. Effect of metalloporphyrins on red autofluorescence from oral bacteria. Eur. J. Oral Sci. 2013, 121, 156–161. [Google Scholar] [CrossRef]
- Higham, S.M.; Pender, N.; Jong, E.D.J.D.; Smith, P.W. Application of biophysical technologies in dental research. J. Appl. Phys. 2009, 105, 102048. [Google Scholar] [CrossRef] [Green Version]
- Bjurshammar, N.; Johannsen, A.; Buhlin, K.; Tranæus, S. On the red fluorescence emission of Aggregatibacter actinomycetemcomitans. Open J. Stomatol. 2012, 2, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Richards-Kortum, R.; Sevick-Muraca, E. Quantitative optical spectroscopy for tissue diagnosis. Annu. Rev. Phys. Chem. 1996, 47, 555–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monici, M. Cell and tissue autofluorescence research and diagnostic applications. Biotechnol. Annu. Rev. 2005, 11, 227–256. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.A.; Kang, S.M.; Kim, S.Y.; Kim, J.S.; Kim, J.B.; Jeong, S.H. Fluorescence change of Fusobacterium nucleatum due to Porphyromonas gingivalis. J. Microbiol. 2018, 56, 628–633. [Google Scholar] [CrossRef] [PubMed]

















| Plaque Disclosing Agent | Autofluorescent Imaging Technology | |
|---|---|---|
| Principle | Adsorption | Fluorescence |
| Test duration | 5−10 min | Real-time < 1 ms |
| After the test | Aesthetic issues | No change |
| Gram-negative bacterial identification | No | Yes |
| Relative thickness of the plaque | Yes | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.-J.; Wang, B.-W.; Yang, C.-M.; Wu, C.-Y.; Ou-Yang, M. Autofluorescence Detection Method for Dental Plaque Bacteria Detection and Classification: Example of Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and Streptococcus mutans. Dent. J. 2021, 9, 74. https://0-doi-org.brum.beds.ac.uk/10.3390/dj9070074
Yan Y-J, Wang B-W, Yang C-M, Wu C-Y, Ou-Yang M. Autofluorescence Detection Method for Dental Plaque Bacteria Detection and Classification: Example of Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and Streptococcus mutans. Dentistry Journal. 2021; 9(7):74. https://0-doi-org.brum.beds.ac.uk/10.3390/dj9070074
Chicago/Turabian StyleYan, Yung-Jhe, Bo-Wen Wang, Chih-Man Yang, Ching-Yi Wu, and Mang Ou-Yang. 2021. "Autofluorescence Detection Method for Dental Plaque Bacteria Detection and Classification: Example of Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and Streptococcus mutans" Dentistry Journal 9, no. 7: 74. https://0-doi-org.brum.beds.ac.uk/10.3390/dj9070074