Mesenchymal Stromal Cells Enhance Vascularization and Epithelialization within 7 Days after Gingival Augmentation with Collagen Matrices in Rabbits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Animals
2.2. Cell Culture
2.3. Collagen Matrices
2.4. Matrices Implantation and Postoperative Monitoring
2.5. Histological Examination
2.6. Immunohistochemistry
2.7. Microscopy
2.8. Statistics
3. Results
3.1. Clinical View of the Postoperative Condition of the Gingiva
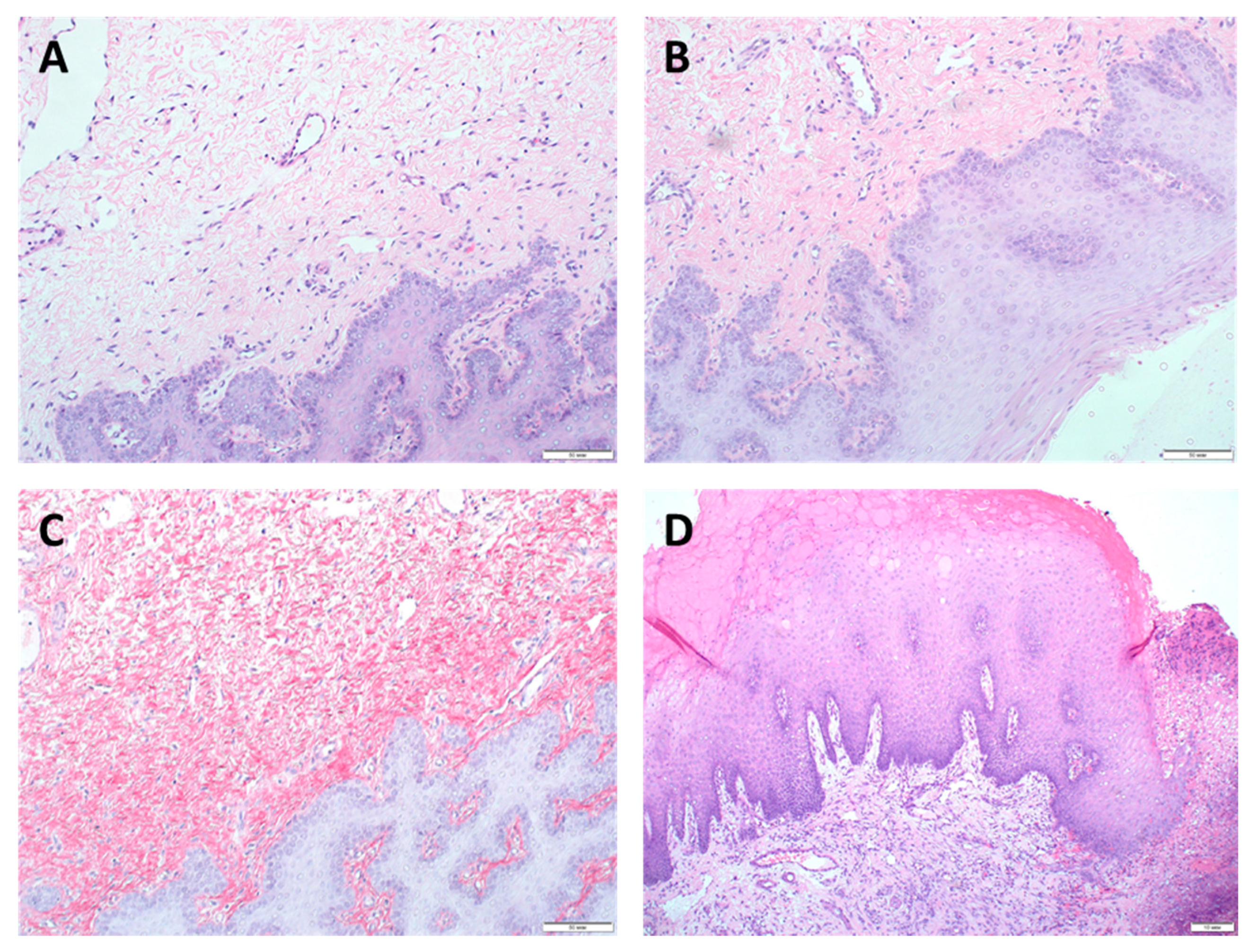
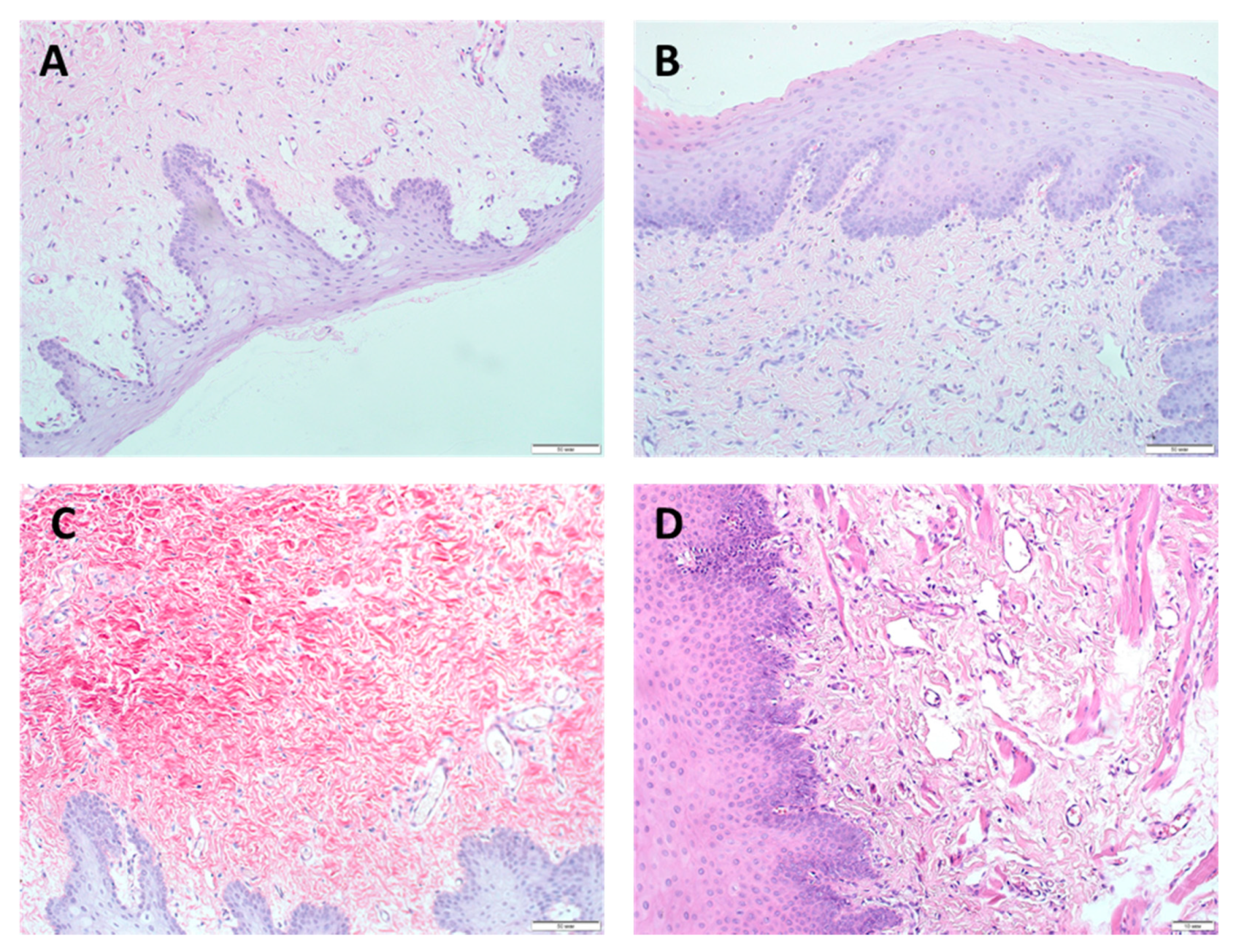
3.2. Morphological Studies
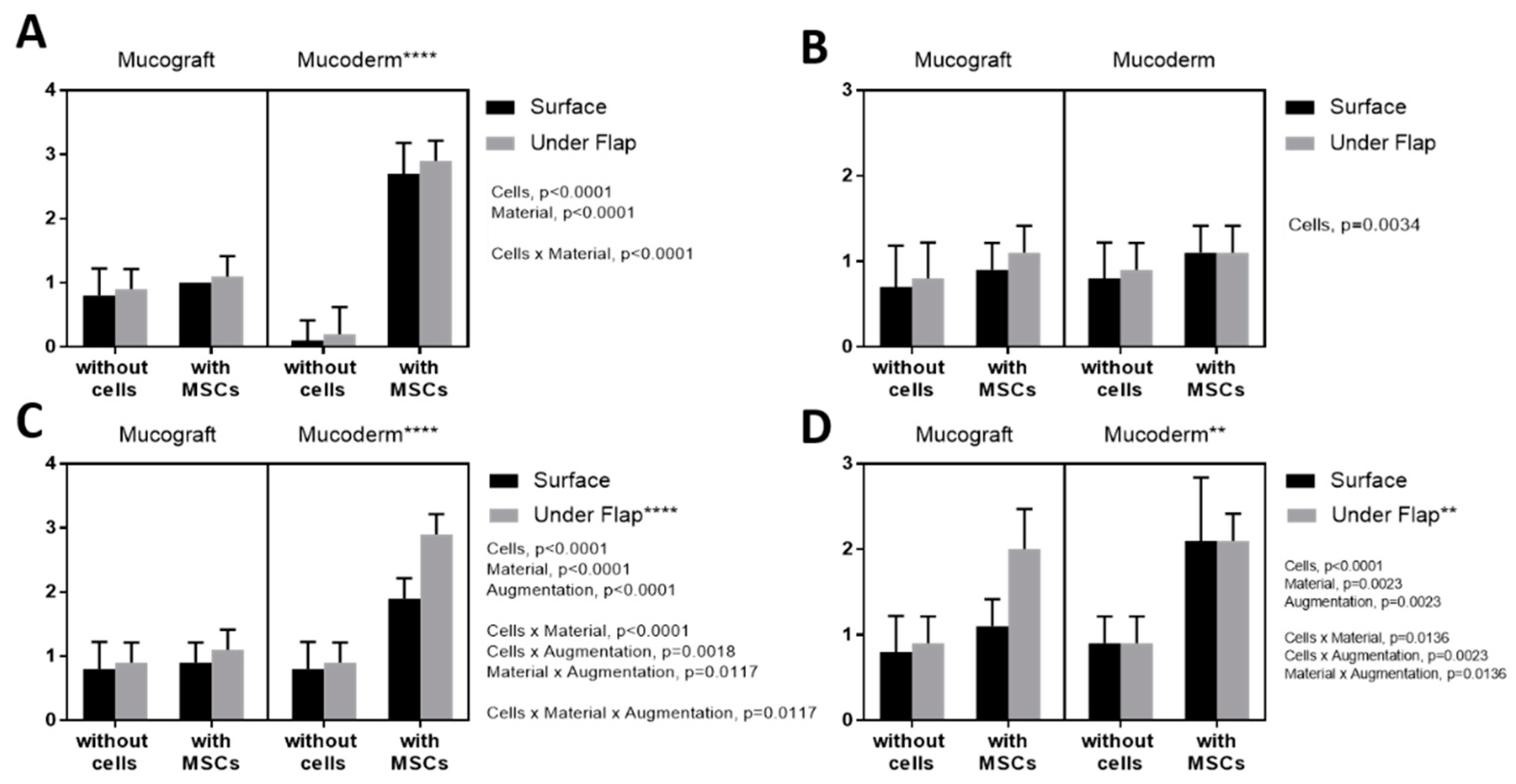
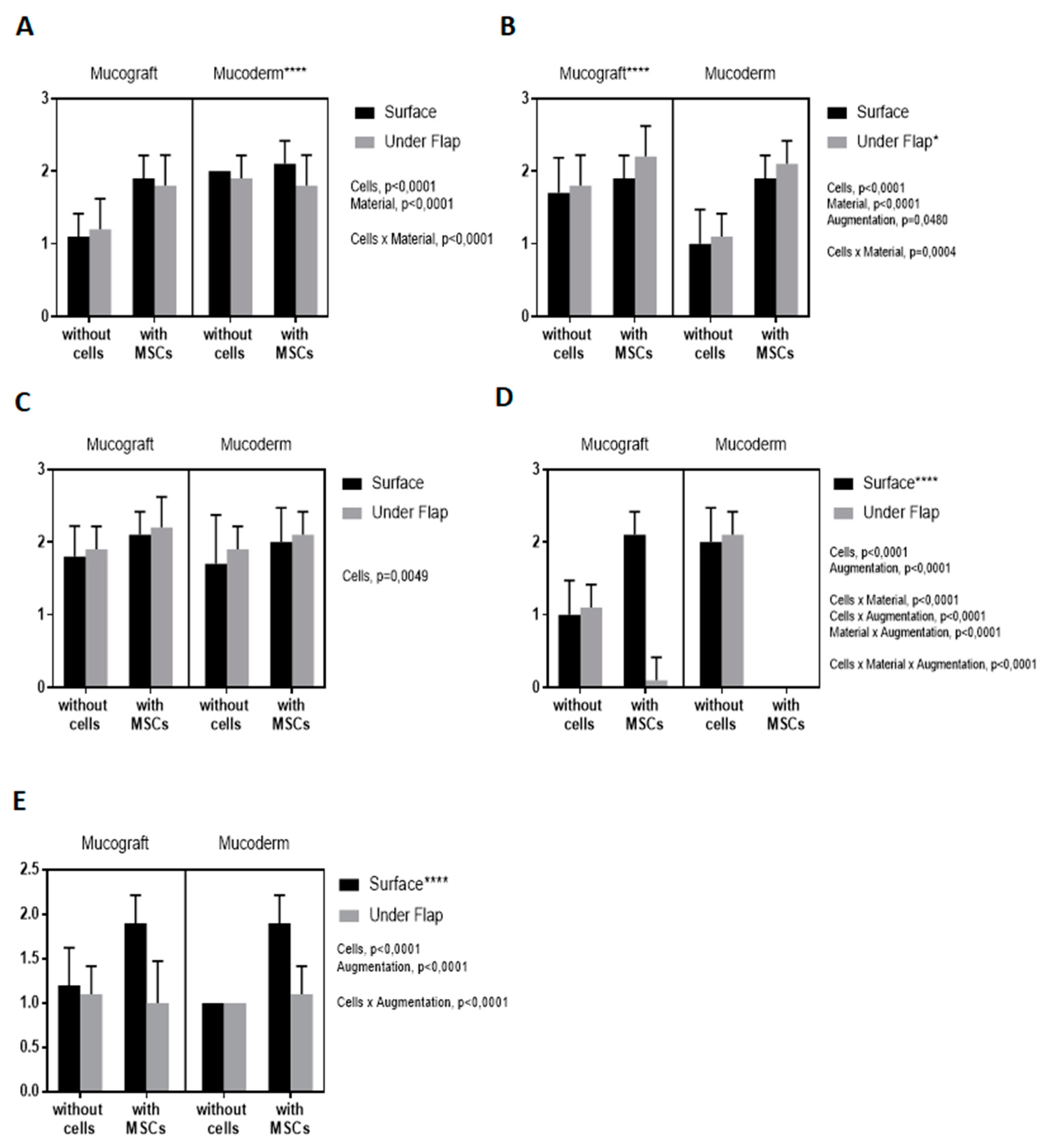
3.3. Epithelialization Assessment
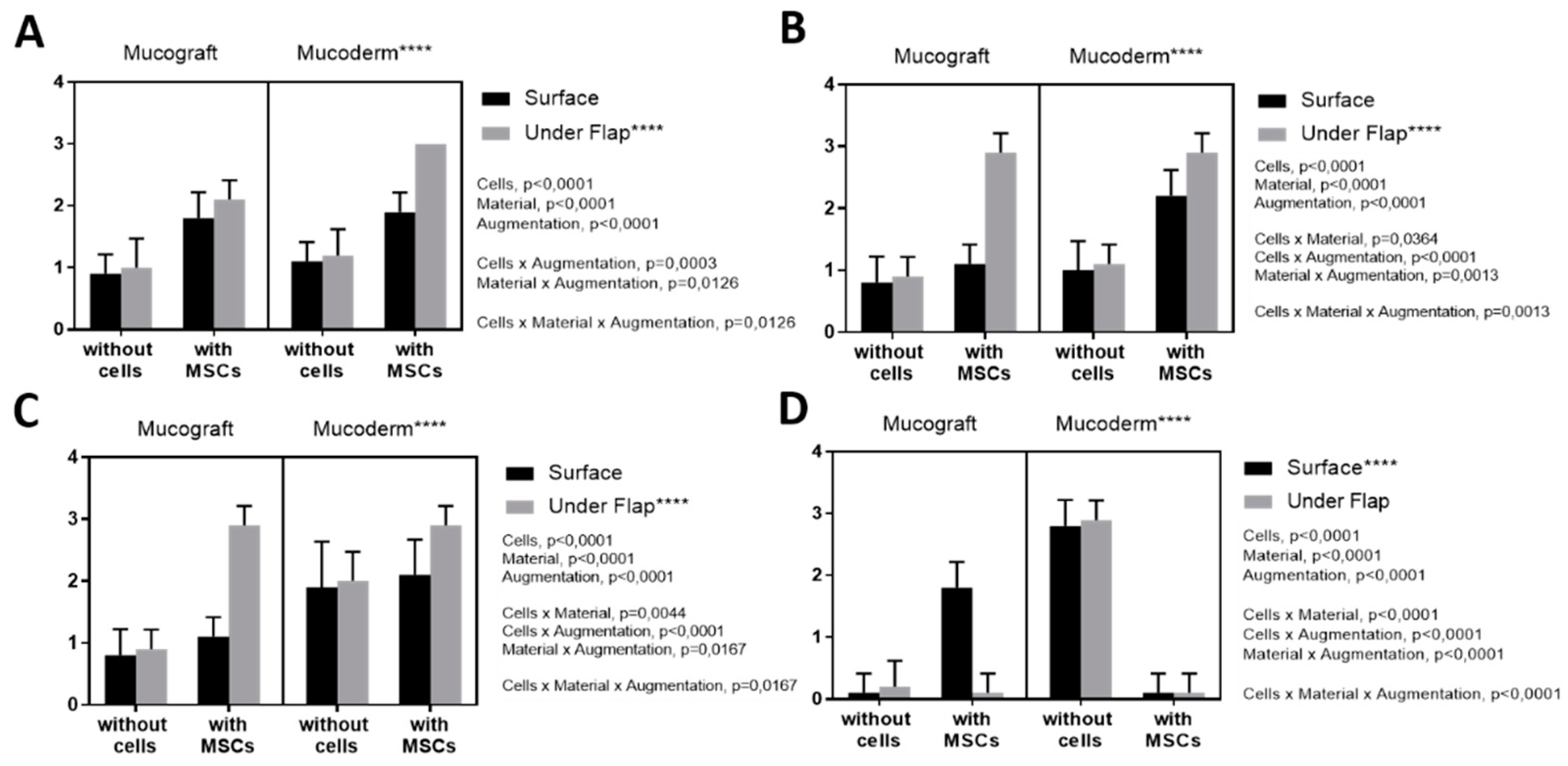
3.4. Angiogenesis Assessment
3.5. Fibroblast Response Assessment
3.6. Lymphoid Infiltration Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cosyn, J.; Hooghe, N.; De Bruyn, H. A systematic review on the frequency of advanced recession following single immediate implant treatment. J. Clin. Periodontol. 2012, 39, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Kasaj, A. Gingival recession coverage using soft tissue substitutes. Clin. Dent. Rev. 2019, 3, 23. [Google Scholar] [CrossRef]
- Xiao, Y.; Qian, H.; Young, W.G.; Bartold, P.M. Tissue engineering for bone regeneration using differentiated alveolar bone cells in collagen scaffolds. Tissue Eng. 2003, 9, 1167–1177. [Google Scholar] [CrossRef]
- Nakahara, T.; Nakamura, T.; Kobayashi, E.; Inoue, M.; Shigeno, K.; Tabata, Y.; Eto, K.; Shimizu, Y. Novel approach to regeneration of periodontal tissues based on in situ tissue engineering: Effects of controlled release of basic fibroblast growth factor from a sandwich membrane. Tissue Eng. 2003, 9, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Egusa, H.; Sonoyama, W.; Nishimura, M.; Atsuta, I.; Akiyama, K. Stem cells in dentistry–part I: Stem cell sources. J. Prosthodont. Res. 2012, 56, 151–165. [Google Scholar] [CrossRef] [Green Version]
- Wolf, J.; Farré-Guasch, E.; Sándor, G.K.; Gibbs, S.; Jager, D.J.; Forouzanfar, T. Soft tissue augmentation techniques and materials used in the oral cavity: An overview. Implant. Dent. 2016, 25, 427–434. [Google Scholar] [CrossRef]
- Sangiorgio, J.P.M.; Neves, F.L.D.S.; Dos Santos, M.R.; França-Grohmann, I.L.; Casarin, R.C.V.; Casati, M.Z.; Santamaria, M.; Sallum, E.A. Xenogenous Collagen Matrix and/or Enamel Matrix Derivative for Treatment of Localized Gingival Recessions: A Randomized Clinical Trial. Part I: Clinical Outcomes. J. Periodontol. 2017, 88, 1309–1318. [Google Scholar] [CrossRef]
- Kasaj, A.; Levin, L.; Stratul, S.-I.; Götz, H.; Schlee, M.; Rütters, C.B.; Konerding, M.A.; Ackermann, M.; Willershausen, B.; Pabst, A.M. The influence of various rehydration protocols on biomechanical properties of different acellular tissue matrices. Clin. Oral Investig. 2016, 20, 1303–1315. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). 1 May 2020. Identifier: NCT03667105. Evaluation of Two Types of Matrix (Mucograft® and Mucoderm®) Associated with Coronally Advanced Flap. Available online: https://clinicaltrials.gov/ct2/show/NCT03667105 (accessed on 1 May 2021).
- Zanwar, K.; Bhongade, M.L.; Ganji, K.; Koudale, S.B.; Gowda, P. Comparative evaluation of efficacy of stem cells in combination with PLA/PGA membrane versus sub-epithelial connective tissue for the treatment of multiple gingival recession defects: A clinical study. J. Stem Cells 2014, 9, 253–267. [Google Scholar] [PubMed]
- Venkataiah, V.S.; Handa, K.; Njuguna, M.M.; Hasegawa, T.; Maruyama, K.; Nemoto, E.; Yamada, S.; Sugawara, S.; Lu, L.; Takedachi, M.; et al. Periodontal regeneration by allogeneic transplantation of adipose tissue derived multi-lineage progenitor stem cells in vivo. Sci. Rep. 2019, 9, 921. [Google Scholar] [CrossRef]
- Li, J.; Xu, S.-Q.; Zhang, K.; Zhang, W.-J.; Liu, H.-L.; Xu, Z.; Li, H.; Lou, J.-N.; Ge, L.-H.; Xu, B.-H. Treatment of gingival defects with gingival mesenchymal stem cells derived from human fetal gingival tissue in a rat model. Stem Cell Res. Ther. 2018, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Yamamoto, Y.; Xiao, Z.; Ochiya, T. The immunomodulatory functions of mesenchymal stromal/stem cells mediated via paracrine activity. J. Clin. Med. 2019, 8, 1025. [Google Scholar] [CrossRef] [Green Version]
- Balyasin, M.; Baranovsky, D.; Demchenko, A.; Fayzullin, A.; Krasilnikova, O.; Klabukov, I.; Krasheninnikov, M.E.; Lyundup, A.V.; Parshin, V.D. Experimental orthotopic implantation of the tissue-engineered graft of trachea based on devitalized scaffold seeded with mesenchymal and epithelial cells. Vestn. Transplant. Isk. Org. 2019, 21, 96–107. [Google Scholar] [CrossRef]
- Pabst, A.M.; Müller, W.E.G.; Ackermann, M. Three-dimensional scanning electron microscopy of maxillofacial biomaterials. Br. J. Oral Maxillofac. Surg. 2017, 55, 736–739. [Google Scholar] [CrossRef]
- Pabst, A.M.; Wagner, W.; Kasaj, A.; Gebhardt, S.; Ackermann, M.; Astolfo, A.; Marone, F.; Haberthur, D.; Enzmann, F.; Konerding, M.A. Synchrotron-based X-ray tomographic microscopy for visualization of three-dimensional collagen matrices. Clin. Oral Investig. 2015, 19, 561–564. [Google Scholar] [CrossRef]
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.; Giannobile, W.V. Extracellular matrix-based scaffolding technologies for periodontal and peri-implant soft tissue regeneration. J. Periodontol. 2020, 91, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.M.; Schlegel, K.A.; Gammel, L.; Moest, T. Gingiva thickening with a porcine collagen matrix in a preclinical dog model: Histological outcomes. J. Clin. Periodontol. 2019, 46, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Tassi, S.A.; Sergio, N.Z.; Misawa, M.Y.O.; Villar, C.C. Efficacy of stem cells on periodontal regeneration: Systematic review of preclinical studies. J. Periodontal Res. 2017, 52, 793–812. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, P.; Kornacker, M.; Mehlhorn, A.; Seckinger, A.; Vohrer, J.; Schmal, H.; Kasten, P.; Eckstein, V.; Südkamp, N.P.; Krause, U. Comparison of immunological properties of bone marrow stromal cells and adipose tissue–derived stem cells before and after osteogenic differentiation in vitro. Tissue Eng. 2007, 13, 111–121. [Google Scholar] [CrossRef]
- Marini, L.; Rojas, M.A.; Sahrmann, P.; Aghazada, R.; Pilloni, A. Early Wound Healing Score: A system to evaluate the early healing of periodontal soft tissue wounds. J. Periodontal Implant. Sci. 2018, 48, 274–283. [Google Scholar] [CrossRef]
- Pippi, R. Post-surgical clinical monitoring of soft tissue wound healing in periodontal and implant surgery. Int. J. Med. Sci. 2017, 14, 721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.E.; Ayoub, N.; Agrawal, D.K. Mesenchymal stem cells and cutaneous wound healing: Novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res. Ther. 2016, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.C.; Martínez, C.; Martínez, J.; McCulloch, C.A. Role of fibroblast populations in periodontal wound healing and tissue remodeling. Front. Physiol. 2019, 10, 270. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Liu, D.; Duan, F.; Wei, Y. Effect of mesenchymal stem cell conditioned medium on human gingival fibroblast proliferation and collagen synthesis. Int. J. Clin. Exp. Med. 2017, 10, 15244–15249. [Google Scholar]
- Zhou, X.; Jin, N.; Wang, F.; Chen, B. Mesenchymal stem cells: A promising way in therapies of graft-versus-host disease. Cancer Cell Int. 2020, 20, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sémont, A.; Demarquay, C.; Bessout, R.; Durand, C.; Benderitter, M.; Mathieu, N. Mesenchymal stem cell therapy stimulates endogenous host progenitor cells to improve colonic epithelial regeneration. PLoS ONE 2013, 8, e70170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasulov, M.F.; Vasil’Chenkov, A.V.; Onishchenko, N.A.; Krasheninnikov, M.E.; Kravchenko, V.I.; Gorshenin, T.L.; Pidtsan, R.E.; Potapov, I.V. First experience in the use of bone marrow mesenchymal stem cells for the treatment of a patient with deep skin burns. Bull. Exp. Biol. Med. 2005, 139, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Kitoko, J.Z.; De Castro, L.L.; Nascimento, A.P.; Abreu, S.C.; Cruz, F.F.; Arantes, A.C.; Xisto, D.G.; Martins, M.A.; Morales, M.M.; Rocco, P.R.M.; et al. Therapeutic administration of bone marrow-derived mesenchymal stromal cells reduces airway inflammation without up-regulating Tregs in experimental asthma. Clin. Exp. Allergy 2018, 48, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Maksimova, N.; Krasheninnikov, M.; Zhang, Y.; Ponomarev, E.; Pomytkin, I.; Mel’Nichenko, G.; Lyundup, A. Early passage autologous mesenchymal stromal cells accelerate diabetic wound re-epithelialization: A clinical case study. Cytotherapy 2017, 19, 1548–1550. [Google Scholar] [CrossRef]

| Group | Subgroups | |
|---|---|---|
| Without Cells (W) | Injection of MSCs (I) | |
| SG (n = 3) | SGW Subgroup: Surface augmentation (S) Mucograft (G) | SGI Subgroup: Surface augmentation (S) Mucograft (G) |
| SD (n = 3) | SDW Subgroup: Surface augmentation (S) Mucoderm (D) | SDI Subgroup: Surface augmentation (S) Mucoderm (D) |
| UG (n = 3) | UGW Subgroup: Augmentation under the flap (U) Mucograft (G) | UGI Subgroup: Augmentation under the flap (U) Mucograft (G) |
| UD (n = 3) | UDW Subgroup: Augmentation under the flap (U) Mucoderm (D) | UDI Subgroup: Augmentation under the flap (U) Mucoderm (D) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulakov, A.; Kogan, E.; Brailovskaya, T.; Vedyaeva, A.; Zharkov, N.; Krasilnikova, O.; Krasheninnikov, M.; Baranovskii, D.; Rasulov, T.; Klabukov, I. Mesenchymal Stromal Cells Enhance Vascularization and Epithelialization within 7 Days after Gingival Augmentation with Collagen Matrices in Rabbits. Dent. J. 2021, 9, 101. https://0-doi-org.brum.beds.ac.uk/10.3390/dj9090101
Kulakov A, Kogan E, Brailovskaya T, Vedyaeva A, Zharkov N, Krasilnikova O, Krasheninnikov M, Baranovskii D, Rasulov T, Klabukov I. Mesenchymal Stromal Cells Enhance Vascularization and Epithelialization within 7 Days after Gingival Augmentation with Collagen Matrices in Rabbits. Dentistry Journal. 2021; 9(9):101. https://0-doi-org.brum.beds.ac.uk/10.3390/dj9090101
Chicago/Turabian StyleKulakov, Anatoliy, Evgenia Kogan, Tatiana Brailovskaya, Anna Vedyaeva, Nickolay Zharkov, Olga Krasilnikova, Mikhail Krasheninnikov, Denis Baranovskii, Timur Rasulov, and Ilya Klabukov. 2021. "Mesenchymal Stromal Cells Enhance Vascularization and Epithelialization within 7 Days after Gingival Augmentation with Collagen Matrices in Rabbits" Dentistry Journal 9, no. 9: 101. https://0-doi-org.brum.beds.ac.uk/10.3390/dj9090101







