Nutritional Characteristics of Black Lentil from Soleto: A Single-Flower Vetch Landrace of Apulia Region (Southern Italy)
Abstract
:1. Introduction
2. Materials and Methods
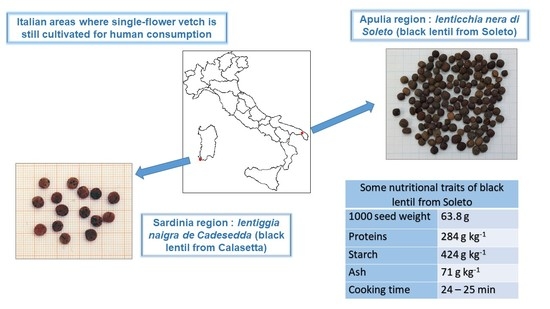
2.1. Plant Materials
2.2. Seed Composition
2.3. Culinary Traits
3. Results and Discussion
3.1. Seed Morphology
3.2. Seed Composition
3.3. Total Phenolic Componds
3.4. Culinary Traits
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuller, D.Q.; Willcox, G.; Allaby, R.G. Cultivation and domestication had multiple origins: Arguments against the core area hypothesis for the origins of agriculture in the Near East. World Archaeol. 2011, 43, 628–652. [Google Scholar] [CrossRef]
- Valamoti, S.M. Plant food ingredients and ‘recipes’ from Prehistoric Greece: The archaeobotanical evidence. In Plants and Culture: Seeds of the Cultural Heritage of Europe; Morel, J.-P., Mercuri, A.M., Eds.; Studio, tutela e fruizione dei Beni Culturali; Edipuglia: Bari, Italy, 2009; pp. 25–38. [Google Scholar]
- Mikić, A.; Mihailović, V.; Ćupina, B.; Đorđević, V.; Milić, D.; Duc, G.; Stoddard, F.L.; Lejeune-Hénaut, I.; Marget, P.; Hanocq, E. Achievements in breeding autumn-sown annual legumes for temperate regions with emphasis on the continental Balkans. Euphytica 2011, 180, 57–67. [Google Scholar] [CrossRef]
- Rubiales, D.; Mikić, A. Introduction: Legumes in Sustainable Agriculture. Crit. Rev. Plant Sci. 2015, 34, 2–3. [Google Scholar] [CrossRef] [Green Version]
- Mikić, A. Presence of vetches (Vicia spp.) in agricultural and wild floras of ancient Europe. Genet. Resour. Crop. Evol. 2016, 63, 745–754. [Google Scholar] [CrossRef]
- Aura, J.E.; Carrión, Y.; Estrelles, E.; Jordà, G.P. Plant economy of hunter-gatherer groups at the end of the last Ice Age: Plant macroremains from the cave of Santa Maira (Alacant, Spain) ca. 12000–9000 b.p. Veg. Hist. Archaeobotany 2005, 14, 542–550. [Google Scholar] [CrossRef]
- Berger, J.; Robertson, L.; Cocks, P. Agricultural potential of Mediterranean grain and forage legumes: 2) Anti-nutritional factor concentrations in the genus Vicia. Genet. Resour. Crop. Evol. 2003, 50, 201–212. [Google Scholar] [CrossRef]
- Sadeghi, G.; Pourreza, J.; Samei, A.; Rahmani, H. Chemical composition and some anti-nutrient content of raw and processed bitter vetch (Vicia ervilia) seed for use as feeding stuff in poultry diet. Trop. Anim. Health Prod. 2009, 41, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Uzun, A.; Güçer, S.; Açıkgöz, E. Common Vetch (Vicia sativa L.) Germplasm: Correlations of Crude Protein and Mineral Content to Seed Traits. Plant Foods Hum. Nutr. 2011, 66, 254–260. [Google Scholar] [CrossRef]
- Francis, C.M.; Enneking, D.; Abd El Moneim, A.M. When and where will vetches have an impact as grain legumes? In Linking Research and Marketing Opportunities for Pulses in the 21st Century, Proceedings of the Third International Food Legume Research Conference, Adelaide, Australia, 22–26 September 1997; Current Plant Science and Biotechnology in Agriculture; Knight, R., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; Volume 34, pp. 671–683. [Google Scholar]
- Pinela, J.; Carvalho, A.M.; Ferreira, I.C. Wild edible plants: Nutritional and toxicological characteristics, retrieval strategies and importance for today’s society. Food Chem. Toxicol. 2017, 110, 165–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peña-Chocarro, L.; Jordà, G.P.; Alonso, N.; Antolín, F.; Teira-Brión, A.; Tereso, J.P.; Moya, E.M.M.; Reyes, D.L. Roman and medieval crops in the Iberian Peninsula: A first overview of seeds and fruits from archaeological sites. Quat. Int. 2019, 499, 49–66. [Google Scholar] [CrossRef]
- Bruni, A. Breve Ragguaglio Dell’agricoltura E Pastorizia Del Regno di Napoli; Filiatre-Sebezio: Napoli, Italy, 1845; p. 86. [Google Scholar]
- De Cesare, C. Delle Condizioni Economiche E Morali Delle Classi Agricole Nelle Tre Provincie di Puglia; Presso T. Guerrero e C.: Napoli, Italy, 1859; p. 26. [Google Scholar]
- Castelli, G. La coltivazione della lenticchia in provincia di Bari. In La Propaganda Agricola XIII; n. 2 February 1935; G. Laterza & Figli: Bari, Italy, 1935. [Google Scholar]
- Maly, R.; Hammer, K.; Lehamann, C.O. Sammlung pflanzlicher genetischer Ressourcen in Sűditalien-ein Reisebericht aus dem Jahre 1950 mit Bemerkungen zum Schicksal der Landsorten “in situ" und in der Genbank. Kulturpflanze 1987, 35, 109–134. [Google Scholar] [CrossRef]
- Istituto Superiore per la Protezione e la Ricerca Ambientale (ISPRA). Frutti Dimenticati e Biodiversità Recuperate; Quaderni Natura e Biodiversità: Roma, Italy, 2013; p. 34. [Google Scholar]
- Laghetti, G.; Piergiovanni, A.; Galasso, I.; Hammer, K.; Perrino, P. Single-flowered vetch (Vicia articulata Hornem.): A relic crop in Italy. Genet. Resour. Crop. Evol. 2000, 47, 461–465. [Google Scholar] [CrossRef]
- Accogli, R.; Dimitri, G.; Marchiori, S. Lenticchia nera di Soleto: Storia locale di un legume minore. In Proceedings of the IX Biodiversity National Congress, Bari, Italy, 5–7 September 2012; Volume II, pp. 262–266. [Google Scholar]
- Piergiovanni, A. The evolution of lentil (Lens culinaris Medik.) cultivation in Italy and its effects on the survival of autochthonous populations. Genet. Resour. Crop. Evol. 2000, 47, 305–314. [Google Scholar] [CrossRef]
- AOAC, Association of Official Agricultural Chemists. Official Methods of Analysis, 11th ed.; AOAC: Washington, DC, USA, 1970. [Google Scholar]
- García-Alonso, A.; Goñi, I.; Saura-Calixto, F. Resistant starch and potential glycaemic index of raw and cooked legumes (lentils, chickpeas and beans). Z. Lebens Unters A 1998, 206, 284–287. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Lai, H.-M. Bioactive Compounds in Legumes and Their Germinated Products. J. Agric. Food Chem. 2006, 54, 3807–3814. [Google Scholar] [CrossRef]
- Della Gatta, C.; Piergiovanni, A.R.; Perrino, P. An improved method for the determination of trypsin inhibitor levels in legumes. Lebens-Wiss. Technol. 1988, 21, 315–318. [Google Scholar]
- Piergiovanni, A.; Cerbino, D.; Della Gatta, C. Diversity in seed quality traits of common bean (Phaseolus vulgaris L.) populations from Basilicata (Southern Italy). Plant Breed. 2000, 119, 513–516. [Google Scholar] [CrossRef]
- Costa, G.E.D.A.; Queiroz-Monici, K.D.S.; Reis, S.M.P.M.; de Oliveira, A.C. Chemical composition, dietary fibre and resistant starch contents of raw and cooked pea, common bean, chickpea and lentil legumes. Food Chem. 2006, 94, 327–330. [Google Scholar] [CrossRef]
- Vioque, R.S.; Prado, I.C.; Gil, F.F.; Alvear, M.J.G.; Pascual, M.D.L.M.; Conde, M.F.R. Contents of total protein, L-canavanine and condensed tannins of the one-flowered vetch (Vicia articulata Hornem.) collection of the Bank of Plant Germplasm of Cuenca (Spain). Genet. Resour. Crop. Evol. 2008, 55, 949–957. [Google Scholar] [CrossRef]
- Piergiovanni, A.R.; Taranto, G. Simple and rapid method for the differentiation of Lens culinaris Medik. by false lentil species. J. Agric. Food Chem. 2005, 53, 6593–6597. [Google Scholar] [CrossRef]
- Pastor-Cavada, E.; Juan, R.; Pastor, J.E.; Alaiz, M.; Vioque, J. Nutritional Characteristics of Seed Proteins in 28 Vicia Species (Fabaceae) from Southern Spain. J. Food Sci. 2011, 76, C1118–C1124. [Google Scholar] [CrossRef]
- Khazaei, H.; Subedi, M.; Nickerson, M.; Martínez-Villaluenga, C.; Frias, J.; Vandenberg, A. Seed Protein of Lentils: Current Status, Progress, and Food Applications. Foods 2019, 8, 391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piergiovanni, A.R.; Galasso, I.; Lioi, L. Protease inhibitors in Phaseolus spp. Seeds. In Seed Proteins. Biochemistry, Functional Properties and Health Benefits; Wilson, D.G., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2017; pp. 41–59. [Google Scholar]
- Piergiovanni, A.R.; Pignone, D. Effect of year-to-year variation and genotype on trypsin inhibitor level in common bean (Phaseolus vulgaris L.) seeds. J. Sci. Food Agric. 2003, 83, 473–476. [Google Scholar] [CrossRef]
- Guillamón, E.; Pedrosa, M.M.; Burbano, C.; Cuadrado, C.; Sánchez, M.D.C.; Muzquiz, M. The trypsin inhibitors present in seed of different grain legume species and cultivar. Food Chem. 2008, 107, 68–74. [Google Scholar] [CrossRef]
- Pisulewska, E.; Pisulewski, P. Trypsin inhibitor activity of legume seeds (peas, chickling vetch, lentils, and soya beans) as affected by the technique of harvest. Anim. Feed. Sci. Technol. 2000, 86, 261–265. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [Green Version]
- Barroso, M.R.; Barros, L.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Fernandes, I.P.; Barreiro, M.F.; Ferreira, I.C. Exploring the antioxidant potential of Helichrysum stoechas (L.) Moench phenolic compounds for cosmetic applications: Chemical characterization, microencapsulation and incorporation into a moisturizer. Ind. Crop. Prod. 2014, 53, 330–336. [Google Scholar] [CrossRef] [Green Version]
- Martins, A.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Fernandes, I.P.; Barreiro, F.; Ferreira, I.C.F.R. Phenolic extracts of Rubus ulmifolius Schott flowers: Characterization, microencapsulation and incorporation into yogurts as nutraceutical sources. Food Funct. 2014, 5, 1091–1100. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition and antioxidant potential of grain legume seeds: A review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Miano, A.C.; Augusto, P.E.D. The Hydration of Grains: A Critical Review from Description of Phenomena to Process Improvements. Compr. Rev. Food Sci. Food Saf. 2018, 17, 352–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiesinger, J.A.; Cichy, K.A.; Glahn, R.P.; Grusak, M.A.; Brick, M.A.; Thompson, H.J.; Tako, E. Demonstrating a Nutritional Advantage to the Fast-Cooking Dry Bean (Phaseolus vulgaris L.). J. Agric. Food Chem. 2016, 64, 8592–8603. [Google Scholar] [CrossRef] [PubMed]


| Seed Trait | lenticchia nera (Black Lentil) | lentil from Altamura | cv. Beluga |
|---|---|---|---|
| 1000 seed weight (g) | 63.8 ± 0.219 a | 59.1 ± 0.062 a | 23.8 ± 1.09 b |
| Protein (g/100 gss) | 28.4 ± 0.312 a | 24.4 ± 0.290 b | 25.0 ± 0.252 b |
| Starch (g/100 gss) | 42.4 ± 1.041 a | 50.2 ± 1.202 b | 54.1 ± 1.604 b |
| Ash (g/100 gss) | 7.1 ± 0.081 a | 6.5 ± 0.068 b | 6.7 ± 0.092 b |
| TPC whole seeds (mg GAE/gss) | 6.02 ± 0.181 a | 5.79 ± 0.103 b | 10.87 ± 0.186 c |
| TPC cotyledons (mg GAE/gss) | 2.25 ± 0.111 a | 1.52 ± 0.020 b | 2.02 ± 0.024 c |
| TPC coats (mg GAE/gss) | 68.23 ± 0.570 a | 88.70 ± 1.605 b | 66.14 ± 1.990 a |
| TPC cooked seeds (mg GAE/gss) | 1.21 ± 0.052 a | 1.50 ± 0.085 b | 1.86 ± 0.037 c |
| Trypsin inhibitors (TIU/mgss) | 4.08 ± 0.192 a | 3.71 ± 0.242 a | 3.93 ± 0.190 a |
| Coat (g/100 g) | 9.2 ± 0.046 a | 6.9 ± 0.042 b | 7.3 ± 0.050 c |
| Hydration index (% at 24 h) | 88.0 ± 0.354 a | 97.1 ± 0.177 b | 96.7 ± 0.374 b |
| Swelling index (% at 24 h) | 82.1 ± 0.551 a | 110.0 ± 0.854 b | 80 ± 0.950 a |
| Cooking time (min) | 24–25 * a | 33–35 ** b | 25–26 ** a |
| Single seed weight before cooking (g) | 0.120 ± 0.034 * a | 0.062 ± 0.013 b | 0.024 ± 0.006 c |
| Single seed weight after cooking (g) | 0.15 ± 0.035 a | 0.12 ± 0.018 a | 0.052 ± 0.008 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piergiovanni, A.R. Nutritional Characteristics of Black Lentil from Soleto: A Single-Flower Vetch Landrace of Apulia Region (Southern Italy). Foods 2021, 10, 2863. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10112863
Piergiovanni AR. Nutritional Characteristics of Black Lentil from Soleto: A Single-Flower Vetch Landrace of Apulia Region (Southern Italy). Foods. 2021; 10(11):2863. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10112863
Chicago/Turabian StylePiergiovanni, Angela Rosa. 2021. "Nutritional Characteristics of Black Lentil from Soleto: A Single-Flower Vetch Landrace of Apulia Region (Southern Italy)" Foods 10, no. 11: 2863. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10112863