Antimicrobial Susceptibility of Lactobacillus delbrueckii subsp. lactis from Milk Products and Other Habitats
Abstract
:1. Introduction
| Product | Substrate | Subspecies of Lactobacillus delbrueckii | Country |
|---|---|---|---|
| Cheese | Animal milk | L. delbrueckii subsp. delbrueckii, L. delbrueckii subsp. lactis | Worldwide |
| Dahi | Cow/buffalo milk, starter culture | L. delbrueckii subsp. indicus | India, Nepal, Sri Lanka, Bangladesh, Pakistan |
| Misti dahi (mishti doi, lal dahi, payodhi) | Cow/buffalo milk | L. delbrueckii subsp. bulgaricus | India, Bangladesh |
| Tarag, Khoormog, Airag, Kumys | Cow/yak/goat/mare milk | L. delbrueckii subsp. bulgaricus | Mongolia |
| Yogurt | Animal milk | L. delbrueckii subsp. bulgaricus | Europe, Australia, America |
| Idli | Rice, black gram, or other dehusked pulses | L. delbrueckii | India, Sri Lanka, Malaysia, Singapore |
| Poto poto | Maize | L. delbrueckii | Congo |
| Sourdough | Rye, wheat | L. delbrueckii | Europe, Australia, America |
| Kimchi | Cabbage, green onion, hot pepper, ginger | L. delbrueckii | Korea |
| Kha Nhom Jeen | Rice | L. delbrueckii | Thailand |
| Soibum | Bamboo shoot | L. delbrueckii | India |
| Sunki | Turnip | L. delbrueckii | Japan |
| Tarhana | Wheat flour, yogurt, vegetables, spices | L. delbrueckii | Turkey |
| Tauco | Soybean | L. delbrueckii | Indonesia |
| Tsukemono | Pickled vegetable | L. delbrueckii | Japan |
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Species-Level Identification
2.3. Genome Sequences of the L. delbrueckii Strains
2.4. Average Nucleotide Identities (ANIs)
2.5. Strains’ Phylogeny
2.6. Screening Assemblies for ARGs
2.7. Antibiotic Susceptibility Testing and MC Determination
3. Results
3.1. Taxonomic Assignment and ANIs
3.2. SNP-Based Phylogenetic Relationships of the Strains
3.3. ARGs in the Genome Assemblies
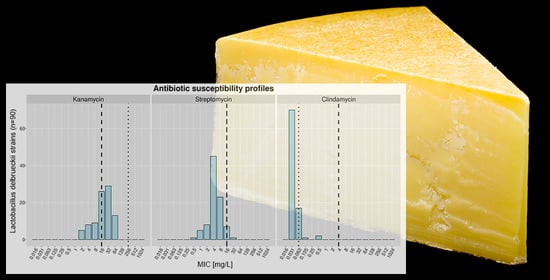
3.4. Antibiotic Susceptibility Profiles and Determination of MCs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourdichon, F.; Alper, I.; Bibiloni, R.; Dubois, A.; Laulund, S.; Miks, M.; Morelli, L.; Yao, S. Inventory of Microbial Food Cultures with Safety Demonstration in Fermented Food Products; Update of the Bulletin of the IDF 455–2012; Bulletin of the International Dairy Federation: Brussels, Belgium, 2018; Volume 495, pp. 1–74. [Google Scholar]
- El Kafsi, H.; Binesse, J.; Loux, V.; Buratti, J.; Boudebbouze, S.; Dervyn, R.; Kennedy, S.; Galleron, N.; Quinquis, B.; Batto, J.-M.; et al. Lactobacillus delbrueckii ssp. lactis and ssp. bulgaricus: A chronicle of evolution in action. BMC Genom. 2014, 15, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Sieuwerts, S.; de Bok, F.A.M.; Hugenholtz, J.; van Hylckama Vlieg, J.E.T. Unraveling Microbial Interactions in Food Fermentations: From Classical to Genomics Approaches. Appl. Environ. Microbiol. 2008, 74, 4997–5007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somerville, V.; Berthoud, H.; Schmidt, R.S.; Bachmann, H.-P.; Meng, Y.H.; Fuchsmann, P.; von Ah, U.; Engel, P. Functional strain redundancy and persistent phage infection in Swiss hard cheese starter cultures. ISME J. 2021, 1–12. [Google Scholar] [CrossRef]
- Schmid, M.; Muri, J.; Melidis, D.; Varadarajan, A.R.; Somerville, V.; Wicki, A.; Moser, A.; Bourqui, M.; Wenzel, C.; Eugster-Meier, E.; et al. Comparative Genomics of Completely Sequenced Lactobacillus helveticus Genomes Provides Insights into Strain-Specific Genes and Resolves Metagenomics Data Down to the Strain Level. Front. Microbiol. 2018, 9, 63. [Google Scholar] [CrossRef]
- Gobbetti, M.; Di Cagno, R.; Calasso, M.; Neviani, E.; Fox, P.F.; De Angelis, M. Drivers that establish and assembly the lactic acid bacteria biota in cheeses. Trends Food Sci. Technol. 2018, 78, 244–254. [Google Scholar] [CrossRef]
- Ghosh, T.; Beniwal, A.; Semwal, A.; Navani, N.K. Mechanistic insights into probiotic properties of lactic acid bacteria associated with ethnic fermented dairy products. Front. Microbiol. 2019, 10, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Bayjanov, J.R.; Renckens, B.; Nauta, A.; Siezen, R.J. The proteolytic system of lactic acid bacteria revisited: A genomic comparison. BMC Genom. 2010, 11, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parente, E.; Cogan, T.M.; Powell, I. Starter cultures: General aspects, chemistry, physics and microbiology. In Cheese, 4th ed.; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Academic Press: London, UK, 2017; pp. 201–226. [Google Scholar] [CrossRef]
- European Food Safety Authority Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F.; et al. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 14: Suitability of taxonomic units notified to EFSA until March 2021. EFSA J. 2021, 19, e06689. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority Panel on Biological Hazards (BIOHAZ); Andreoletti, O.; Budka, H.; Buncic, S.; Colin, P.; Collins, J.D.; De Koeijer, A.; Griffin, J.; Havelaar, A.; Hope, J.; et al. The maintenance of the list of QPS microorganisms intentionally added to food or feed—scientific opinion of the Panel on Biological Hazards. EFSA J. 2008, 6, 923. [Google Scholar] [CrossRef]
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 2018, 169, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, J.R. Horizontal gene transfer in the human gastrointestinal tract: Potential spread of antibiotic resistance genes. Infect. Drug Resist. 2014, 7, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Lerner, A.; Matthias, T.; Aminov, R. Potential Effects of Horizontal Gene Exchange in the Human Gut. Front. Immunol. 2017, 8, 1630. [Google Scholar] [CrossRef] [Green Version]
- Thumu, S.C.R.; Halami, P.M. Conjugal transfer of erm(B) and multiple tet genes from Lactobacillus spp. to bacterial pathogens in animal gut, in vitro and during food fermentation. Food Res. Int. 2018, 116, 1066–1075. [Google Scholar] [CrossRef]
- Herman, L.; Chemaly, M.; Cocconcelli, P.S.; Fernandez, P.; Klein, G.; Peixe, L.; Prieto, M.; Querol, A.; Suarez, J.E.; Sundh, I.; et al. The qualified presumption of safety assessment and its role in EFSA risk evaluations: 15 years past. FEMS Microbiol. Lett. 2018, 366. [Google Scholar] [CrossRef]
- European Food Safety Authority Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.d.L.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; et al. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA J. 2018, 16, 5206. [Google Scholar]
- Hammes, W.P.; Hertel, C. Lactobacillus. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., DeVos, P., Dedysh, S., Hedlund, B., Kämpfer, P., Rainey, F., Trujillo, M.E., Bowman, J.P., Brown, D.R., Glöckner, F.O., et al., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, Bergey’s Manual Trust: New York, NY, USA, 2015; pp. 1–76. [Google Scholar]
- Shani, N.; Oberhaensli, S.; Arias-Roth, E. Antibiotic Susceptibility Profiles of Pediococcus pentosaceus from Various Origins and Their Implications for the Safety Assessment of Strains with Food-Technology Applications. J. Food Prot. 2020, 84, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J.; Reale, A. A Holistic Review on Euro-Asian Lactic Acid Bacteria Fermented Cereals and Vegetables. Microorganisms 2020, 8, 1176. [Google Scholar] [CrossRef]
- Tamang, J.P.; Watanabe, K.; Holzapfel, W.H. Review: Diversity of Microorganisms in Global Fermented Foods and Beverages. Front. Microbiol. 2016, 7, 377. [Google Scholar] [CrossRef] [Green Version]
- De Man, J.C.; Rogosa, M.; Sharpe, M.E. A Medium for the Cultivation of Lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Lüdin, P.; Roetschi, A.; Wüthrich, D.; Bruggmann, R.; Berthoud, H.; Shani, N. Update on Tetracycline Susceptibility of Pediococcus acidilactici Based on Strains Isolated from Swiss Cheese and Whey. J. Food Prot. 2018, 81, 1582–1589. [Google Scholar] [CrossRef]
- Dreier, M.; Berthoud, H.; Shani, N.; Wechsler, D.; Junier, P. SpeciesPrimer: A bioinformatics pipeline dedicated to the design of qPCR primers for the quantification of bacterial species. PeerJ 2020, 8, e8544. [Google Scholar] [CrossRef] [Green Version]
- Low, A.J.; Koziol, A.G.; Manninger, P.A.; Blais, B.; Carrillo, C.D. ConFindr: Rapid detection of intraspecies and cross-species contamination in bacterial whole-genome sequence data. PeerJ 2019, 7, e6995. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 1 June 2021).
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Seppey, M.; Manni, M.; Zdobnov, E.M. BUSCO: Assessing Genome Assembly and Annotation Completeness. In Gene Prediction; Kollmar, M., Ed.; Methods in Molecular Biology; Humana: New York, NY, USA, 2019; Volume 1962, pp. 227–245. [Google Scholar]
- Jain, C.; Rodriguez-R, L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef] [Green Version]
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; et al. gplots: Various R Programming Tools for Plotting Data. Available online: https://CRAN.R-project.org/package=gplots (accessed on 12 October 2021).
- R StudioTeam. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 12 October 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 12 October 2021).
- Shakya, M.; Ahmed, S.A.; Davenport, K.W.; Flynn, M.C.; Lo, C.-C.; Chain, P.S.G. Standardized phylogenetic and molecular evolutionary analysis applied to species across the microbial tree of life. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [Green Version]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Dijkshoorn, L.; Ursing, B.; Ursing, J. Strain, clone and species: Comments on three basic concepts of bacteriology. J. Med Microbiol. 2000, 49, 397–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seemann, T. Abricate. 2020. Available online: https://github.com/tseemann/abricate (accessed on 25 February 2020).
- Feldgarden, M.; Brover, V.; Haft, D.H.; Prasad, A.B.; Slotta, D.J.; Tolstoy, I.; Tyson, G.H.; Zhao, S.; Hsu, C.-H.; McDermott, P.F.; et al. Validating the AMRFinder Tool and Resistance Gene Database by Using Antimicrobial Resistance Genotype-Phenotype Correlations in a Collection of Isolates. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [Green Version]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD 2017: Expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017, 45, D566–D573. [Google Scholar] [CrossRef]
- McArthur, A.G.; Waglechner, N.; Nizam, F.; Yan, A.; Azad, M.A.; Baylay, A.J.; Bhullar, K.; Canova, M.J.; De Pascale, G.; Ejim, L.; et al. The Comprehensive Antibiotic Resistance Database. Antimicrob. Agents Chemother. 2013, 57, 3348–3357. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Padmanabhan, B.R.; Diene, S.M.; Lopez-Rojas, R.; Kempf, M.; Landraud, L.; Rolain, J.-M. ARG-ANNOT, a New Bioinformatic Tool to Discover Antibiotic Resistance Genes in Bacterial Genomes. Antimicrob. Agents Chemother. 2013, 58, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 11.0. 2021. Available online: http://www.eucast.org (accessed on 28 July 2021).
- International Organization for Standardization (ISO). Milk and Milk Products—Determination of the Minimal Inhibitory Concentration (MIC) of Antibiotics Applicable to Bifidobacteria and Non-Enterococcal Lactic Acid Bacteria (LAB); ISO 10932|IDF 223:2010; International Organization for Standardization: Geneva, Switzerland, 2010. [Google Scholar]
- Klare, I.; Konstabel, C.; Müller-Bertling, S.; Reissbrodt, R.; Huys, G.; Vancanneyt, M.; Swings, J.; Goossens, H.; Witte, W. Evaluation of New Broth Media for Microdilution Antibiotic Susceptibility Testing of Lactobacilli, Pediococci, Lactococci, and Bifidobacteria. Appl. Environ. Microbiol. 2005, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnidge, J.; Kahlmeter, G.; Kronvall, G. Statistical characterisation of bacterial wild-type MIC value distributions and the determination of epidemiological cut-off values. Clin. Microbiol. Infect. 2006, 12, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer International Publishing: New York, NY, USA, 2016. [Google Scholar]
- Böckelmann, U.; Dörries, H.-H.; Ayuso-Gabella, M.N.; de Marçay, M.S.; Tandoi, V.; Levantesi, C.; Masciopinto, C.; Van Houtte, E.; Szewzyk, U.; Wintgens, T.; et al. Quantitative PCR Monitoring of Antibiotic Resistance Genes and Bacterial Pathogens in Three European Artificial Groundwater Recharge Systems. Appl. Environ. Microbiol. 2009, 75, 154–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varghese, N.J.; Mukherjee, S.; Ivanova, N.; Konstantinidis, K.T.; Mavrommatis, K.; Kyrpides, N.C.; Pati, A. Microbial species delineation using whole genome sequences. Nucleic Acids Res. 2015, 43, 6761–6771. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, J.L.; Coque, T.M.; Baquero, F. What is a resistance gene? Ranking risk in resistomes. Nat. Rev. Genet. 2014, 13, 116–123. [Google Scholar] [CrossRef]
- Pal, C.; Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. The structure and diversity of human, animal and environmental resistomes. Microbiome 2016, 4, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ksibi, B.; Ktari, S.; Othman, H.; Ghedira, K.; Maalej, S.; Mnif, B.; Abbassi, M.S.; Fabre, L.; Rhimi, F.; Le Hello, S.; et al. Comparison of conventional molecular and whole-genome sequencing methods for subtyping Salmonella enterica serovar Enteritidis strains from Tunisia. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 40, 597–606. [Google Scholar] [CrossRef]
- Schürch, A.; Arredondo-Alonso, S.; Willems, R.; Goering, R. Whole genome sequencing options for bacterial strain typing and epidemiologic analysis based on single nucleotide polymorphism versus gene-by-gene–based approaches. Clin. Microbiol. Infect. 2018, 24, 350–354. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.J.; Lappi, V.; Wolfgang, W.J.; Lapierre, P.; Palumbo, M.J.; Medus, C.; Boxrud, D. Characterization of Foodborne Outbreaks of Salmonella enterica Serovar Enteritidis with Whole-Genome Sequencing Single Nucleotide Polymorphism-Based Analysis for Surveillance and Outbreak Detection. J. Clin. Microbiol. 2015, 53, 3334–3340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.; Zhang, W.; Kwok, L.-Y.; Menghe, B. In vitro evalution of antibiotic resistance of Lactobacillus bulgaricus strains isolated from traditional dairy products. Czech J. Food Sci. 2019, 37, 36–43. [Google Scholar] [CrossRef]
- Cho, G.-S.; Cappello, C.; Schrader, K.; Fagbemigum, O.; Oguntoyinbo, F.A.; Csovcsics, C.; Sch, N.R.; Kabisch, J.; Neve, H.; Bockelmann, W.; et al. Isolation and Characterization of Lactic Acid Bacteria from Fermented Goat Milk in Tajikistan. J. Microbiol. Biotechnol. 2018, 28, 1834–1845. [Google Scholar] [CrossRef]
- Rossetti, L.; Carminati, D.; Zago, M.; Giraffa, G. A Qualified Presumption of Safety approach for the safety assessment of Grana Padano whey starters. Int. J. Food Microbiol. 2009, 130, 70–73. [Google Scholar] [CrossRef]
- D’Aimmo, M.R.; Modesto, M.; Biavati, B. Antibiotic resistance of lactic acid bacteria and Bifidobacterium spp. isolated from dairy and pharmaceutical products. Int. J. Food Microbiol. 2007, 115, 35–42. [Google Scholar] [CrossRef]
- Stefańska, I.; Kwiecień, E.; Jóźwiak-Piasecka, K.; Garbowska, M.; Binek, M.; Rzewuska, M. Antimicrobial susceptibility of lactic acid bacteria strains of potential use as feed additives—The basic safety and usefulness criterion. Front. Vet. Sci. 2021, 9, 687071. [Google Scholar] [CrossRef]




| No. | Subspecies b | Strain ID | Culture Collection a | Origin | Year of Isolation | Original Depositor; Strain Designation | GenBank Assembly Accessions d |
|---|---|---|---|---|---|---|---|
| 1 | LDL | FAM 1200 | ACC | Unknown | Unknown | FAM 1200 | GCA_021135575.1 |
| 2 | LDL | FAM 8520 | ACC | Swiss milk | Unknown | Jimeno, J.; FM 1 | GCA_021135595.1 |
| 3 | LDL | FAM 10980 | ACC | Swiss undefined mixed starter culture | 1979 | Isolini, D.; Lb 101.07 | GCA_021135555.1 |
| 4 | LDL | FAM 10983 | ACC | Swiss undefined mixed starter culture | 1984 | Isolini, D.; Lb 101.10 | GCA_021135485.1 |
| 5 | LDL | FAM 10991 | ACC | Swiss undefined mixed starter culture | 1980 | Isolini, D.; Lb 104.80 | GCA_021135515.1 |
| 6 | LDL | FAM 11021 | ACC | Swiss undefined mixed starter culture | 1982 | Isolini, D.; Lb 115.53 | GCA_021135535.1 |
| 7 | LDL | FAM 11036 | ACC | Swiss undefined mixed starter culture | 1979 | Isolini, D.; Lb 119.17 | GCA_021135475.1 |
| 8 | LDL | FAM 11075 | ACC | Swiss undefined mixed starter culture | 1978 | Isolini, D.; Lb 150.14 | GCA_021135455.1 |
| 9 | LDL | FAM 11108 | ACC | Swiss undefined mixed starter culture | 1983 | Isolini, D.; Lb 157.02 | GCA_021135435.1 |
| 10 | LDL | FAM 11129 | ACC | Swiss undefined mixed starter culture | 1983 | Isolini, D.; Lb 164.35 | GCA_021135405.1 |
| 11 | LDL | FAM 11142 | ACC | Swiss undefined mixed starter culture | 1988 | Isolini, D.; Lb 202.02 | GCA_021135395.1 |
| 12 | LDL | FAM 12062 | ACC | Swiss undefined mixed starter culture | 1983 | Isolini, D.; Lb 302.01 | GCA_021135375.1 |
| 13 | LDL | FAM 12103 | ACC | Swiss undefined mixed starter culture | 1986 | Isolini, D.; Lb 325.13 | GCA_021135355.1 |
| 14 | LDL | FAM 12107 | ACC | Swiss undefined mixed starter culture | 1988 | Isolini, D.; Lb 202.07 | GCA_021135335.1 |
| 15 | LDL | FAM 12109 | ACC | Swiss undefined mixed starter culture | 1988 | Isolini, D.; Lb 202.09 | GCA_021135275.1 |
| 16 | LDL | FAM 18834 | ACC | Swiss milk | 2005 | Unknown; C1 | GCA_021135295.1 |
| 17 | LDL | FAM 19699 | ACC | Swiss Emmental cheese | 1989–1990 | Isolini, D.; 23.10 | GCA_021135315.1 |
| 18 | LDL | FAM 19994 | ACC | Swiss Emmental cheese | 1989–1990 | Isolini, D.; 43.13 | GCA_021135255.1 |
| 19 | LDL | FAM 20408 | ACC | Swiss hard cheese | 1989–1990 | Isolini, D.; LDELA 871-33 | GCA_021135235.1 |
| 20 | LDL | FAM 20544 | ACC | Swiss hard cheese | 1989–1990 | Isolini, D.; LDEBU 927-84 | GCA_021135205.1 |
| 21 | LDL | FAM 21277 | ACC | Swiss undefined mixed starter culture | Unknown | Meyer, J.; 101/100 | GCA_005864055.1 |
| 22 | LDL | FAM 21376 | ACC | Swiss undefined mixed starter culture | Unknown | Meyer, J.; 169/126 | GCA_021135175.1 |
| 23 | LDL | FAM 21745 | ACC | Swiss undefined mixed starter culture | 1979 | Isolini, D.; Lb 101.01 | GCA_021135195.1 |
| 24 | LDL | FAM 21748 | ACC | Swiss undefined mixed starter culture | 1979 | Isolini, D.; Lb 101.56 | GCA_021135155.1 |
| 25 | LDL | FAM 21753 | ACC | Swiss undefined mixed starter culture | 1981 | Isolini, D.; Lb 124.49 | GCA_021135095.1 |
| 26 | LDL | FAM 21754 | ACC | Swiss undefined mixed starter culture | 1982 | Isolini, D.; Lb 153.48 | GCA_021135135.1 |
| 27 | LDL | FAM 21755 | ACC | Swiss undefined mixed starter culture | 1982 | Isolini, D.; Lb 153.08 | GCA_021135105.1 |
| 28 | LDL | FAM 21756 | ACC | Swiss undefined mixed starter culture | 1982 | Isolini, D.; Lb 153.09 | GCA_021135075.1 |
| 29 | LDL | FAM 21768 | ACC | Swiss undefined mixed starter culture | 1982 | Isolini, D.; Lb 153.20 | GCA_021135055.1 |
| 30 | LDL | FAM 21769 | ACC | Swiss undefined mixed starter culture | 1978 | Isolini, D.; Lb 150.10 | GCA_021135025.1 |
| 31 | LDL | FAM 21781 | ACC | Swiss undefined mixed starter culture | 1981 | Isolini, D.; Lb 124.09 | GCA_021135015.1 |
| 32 | LDL | FAM 21783 | ACC | Swiss undefined mixed starter culture | 1982 | Isolini, D.; Lb 153.27 | GCA_021134955.1 |
| 33 | LDL | FAM 21784 | ACC | Swiss undefined mixed starter culture | 1982 | Isolini, D.; Lb 153.28 | GCA_005864125.1 |
| 34 | LDL | FAM 22091 | ACC | Swiss natural whey culture | 1973 | A50.2 | GCA_021134995.1 |
| 35 | LDL | FAM 22092 | ACC | Swiss natural whey culture | 1977 | A56.1 | GCA_021134975.1 |
| 36 | LDLc | FAM 22093 | ACC | Swiss natural whey culture | 1977 | A66.5 | GCA_021134935.1 |
| 37 | LDL | FAM 22274 | ACC | Swiss natural whey culture | 1968 | A44.2 | GCA_021134915.1 |
| 38 | LDL | FAM 22332 | ACC | Swiss undefined mixed starter culture | Unknown | Weishaupt, C.; 313.1 | GCA_021134885.1 |
| 39 | LDL | FAM 22680 | ACC | Swiss natural whey culture | 1967 | A77.5 | GCA_021134855.1 |
| 40 | LDL | FAM 24199 | ACC | Swiss Tomme cheese | 2017 | Shani, N.; 55/8 | GCA_021134875.1 |
| 41 | LDS (LDL) | CIP 101810 | CIP | Unknown | Unknown | Unknown | GCA_021134835.1 |
| 42 | LDL | CIP 110109 | CIP | Human urine, France | 1976 | Vandekerkove; 103-76 | GCA_021134765.1 |
| 43 | LDL c | CIRM BIA 225 | CIRM | Artisanal lactic starter (for Gruyère de Comté cheese making), Franche-Comté, France | 1963 | Accolas, J.P.; P12 | GCA_021134795.1 |
| 44 | LDL | CIRM BIA 229 | CIRM | Artisanal lactic starter (for Gruyère de Comté cheese making), Franche-Comté, France | 1964 | Accolas, J.P.; H5a | GCA_021134815.1 |
| 45 | LDL | CIRM BIA 230 | CIRM | Cheese (Emmental), Finland | 1968 | CNRZ331 | GCA_021134745.1 |
| 46 | LDL | CIRM BIA 233 | CIRM | Probably a French yoghurt factory | 1984 | Cluzel, P.J.; LT4-G2N | GCA_021134735.1 |
| 47 | LDL | CIRM BIA 234 | CIRM | Probably a French yoghurt factory | 1984 | Cluzel, P.J.; LT4-G18 | GCA_021134695.1 |
| 48 | LDL | CIRM BIA 265 | CIRM | Cheese (Emmental), Finland | 1968 | CNRZ330 | GCA_021134675.1 |
| 49 | LDS (LDL) | CIRM BIA 266 | CIRM | Fermented milk (kefir), Russia | 1971 | Accolas, J.P.; KFA1 | GCA_021134645.1 |
| 50 | LDL | CIRM BIA 267 | CIRM | Lactic starter (for Emmental cheese making), Finland | 1974 | Tybeck, E., LKT; VALIO | GCA_021134615.1 |
| 51 | LDL | CIRM BIA 269 | CIRM | Artisanal lactic starter (for Emmental cheese making), Finland | Unknown | Tybeck, E.; ISL 19 | GCA_021134635.1 |
| 52 | LDL | CIRM BIA 1368 | CIRM | Starter (for Grana Padano cheese making), Piedmont, Italy | 1988 | IMPC Al | GCA_021134595.1 |
| 53 | LDL | CIRM BIA 1372 | CIRM | Yak milk, Nepal | 1996 | Quenee, P.; Np 5t | GCA_021134505.1 |
| 54 | LDL | CIRM BIA 1374 | CIRM | Whey (from Comté), Franche-Comté, France | 1993 | CML10 | GCA_021134545.1 |
| 55 | LDLc | DSM 20072 T | DSMZ | Emmental cheese (country of origin unknown) | Before 22.08.1990 | Snog-Kjaer, A. (Orla-Jensen, S., Thermobacterium lactis No. 10) | GCA_002278095.1 |
| 56 | LDL | DSM 20073 | DSMZ | Saliva (country of origin unknown) | Before 22.08.1990 | Williams, N.; 14-1 | GCA_021134535.1 |
| 57 | LDL | DSM 20076 | DSMZ | Unknown | Before 22.08.1990 | Fred, E.B.; F 59 | GCA_021134495.1 |
| 58 | LDL | DSM 20355 | DSMZ | Unknown | Before 22.08.1990 | McCoy; Ld 5 | GCA_021134475.1 |
| 59 | LDL | NCIMB 7278 | NCIMB | Unknown | Before 01.01.1950 | Dorner, W.; 39 E/K | GCA_021134455.1 |
| 60 | LDL | NCIMB 8011 | NCIMB | Unknown | Before 07.02.1950 | Dorner | GCA_021134375.1 |
| 61 | LDL | NCIMB 8140 | NCIMB | “Ga” starter culture | Before 01.09.1956 | “““Ga””” | GCA_021134435.1 |
| 62 | LDL | NCIMB 8170 | NCIMB | Unknown | Before 1999 | Merck & Co., Inc.; MB 367 | GCA_021134415.1 |
| 63 | LDL | NCIMB 8183 | NCIMB | Unknown | Before 30.11.1950 | McCoy, E.; 326 | GCA_021134395.1 |
| 64 | LDL | NCIMB 8882 | NCIMB | Unknown | Before 01.07.1957 | Winkler, K.C.; 1175 | GCA_021134335.1 |
| 65 | LDL | NCIMB 8964 | NCIMB | Unknown | Before 01.12.1958 | Galloway & Barton-Wright | GCA_021134315.1 |
| 66 | LDL | NCIMB 700280 | NCIMB | Unknown | Before 01.01.1954 | 244 | GCA_021134355.1 |
| 67 | LDLc | NCIMB 700820 | NCIMB | Unknown | Before 01.01.1954 | C808/5 | GCA_021134295.1 |
| 68 | LDL | NCIMB 700860 | NCIMB | Unknown | Before 01.01.1956 | 18/40 | GCA_021134275.1 |
| 69 | LDL | NCIMB 701040 | NCIMB | Italian hard cheese | Before 01.01.1957 | C14/8 | GCA_021134235.1 |
| 70 | LDL | NCIMB 701437 | NCIMB | Unknown | Unknown | Snog-Kjaer, A.; LI 1 | GCA_021134215.1 |
| 71 | LDL | NCIMB 702465 | NCIMB | Dried calve stomachs for Gruyere cheese | Unknown | L24, C57 | GCA_021134255.1 |
| 72 | LDL | NCIMB 702466 | NCIMB | Switzerland | Unknown | Ritter, P.; L26, H14, 1304 | GCA_021134175.1 |
| 73 | LDL | NCIMB 702467 | NCIMB | Sweden | Before 01.01.1981 | Swartling, P.; L27, L39 (WL39) | GCA_021134185.1 |
| 74 | LDL (LDS) | NCIMB 702468 | NCIMB | Distillery | Before 01.01.1961 | Sharpe, M.E.; M2/2 | GCA_021134155.1 |
| 75 | LDL (LDD) | NCIMB 702469 | NCIMB | Distillery | Before 01.01.1961 | Sharpe, M.E.; LE8, M2/3 | GCA_021134135.1 |
| 76 | LDL | FAM 24847 | NC | Commercial cheese starter culture | 2019 | na | GCA_021134095.1 |
| 77 | LDL | FAM 24848 | NC | Commercial cheese starter culture | 2019 | na | GCA_021134115.1 |
| 78 | LDL | FAM 24849 | NC | Commercial cheese starter culture | 2019 | na | GCA_021134075.1 |
| 79 | LDL | FAM 24850 | NC | Commercial cheese starter culture | 2019 | na | GCA_021134035.1 |
| 80 | LDL | FAM 24851 | NC | Commercial cheese starter culture | 2019 | na | GCA_021134055.1 |
| 81 | LDL | FAM 24852 | NC | Commercial cheese starter culture | 2019 | na | GCA_021134005.1 |
| 82 | LDB | FAM 22166 | ACC | Swiss natural whey culture | 1968 | A135.3 | GCA_021133995.1 |
| 83 | LDB | FAM 22754 | ACC | Yoghurt | 1952 | A171.1 | GCA_021133975.1 |
| 84 | LDB | CIRM BIA 657 | CIRM | Fermented milk, Crete, Greece | 1987 | Zourari, A.; ZL071B1 | GCA_021133935.1 |
| 85 | LDB | CIRM BIA 773 | CIRM | Lactic starter (for yoghurt making), Île-de-France, France | 1963 | Chevalier, R.; LT1 | GCA_021133955.1 |
| 86 | LDB | CIRM BIA 860 | CIRM | Lactic starter (for yoghurt making), Rhône-Alpes, France | 1971 | Accolas, J.P.; LAY 1 | GCA_021133915.1 |
| 87 | LDB | CIRM BIA 864 | CIRM | Fermented milk, France | Unknown | IL1609 | GCA_021133885.1 |
| 88 | Lactobacillus johnsonii (LDB) | CIRM BIA 879 | CIRM | Tarag (yoghurt), Mongolia | 1974 | Accolas, J.P. | na |
| 89 | LDB | CIRM BIA 905 | CIRM | Cheese (Boulettes d’Avesne), Nord-Pas-de-Calais, France | 1998 | Quenee, P.; AV2 | GCA_021133875.1 |
| 90 | LDB | CIRM BIA 906 | CIRM | Yak milk, Nepal | 1996 | Quenee, P.; NP 2T | GCA_021133835.1 |
| 91 | LDL (LDB) c | CIRM BIA 1375 | CIRM | Cheese (Morbier, raw milk), Jura, France | 1993 | 10F10 | GCA_021133855.1 |
| 92 | LDB | CIRM BIA 1376 | CIRM | Lactic starter (for yoghurt making), Bulgaria | 1978 | Bouillanne, C. 5 | GCA_021133815.1 |
| 93 | LDB | CIRM BIA 1379 | CIRM | Yoghurt (ewe milk), Crete, Greece | 1987 | Zourari, A.; ZL023A1 | GCA_021133775.1 |
| 94 | LDBc | CIRM BIA 1381 | CIRM | Yoghurt, Bali, Indonesia | 1990 | CNRZ1493 | GCA_021133785.1 |
| 95 | LDB | CIRM BIA 1579 | CIRM | Lactic starter, USA | 1974 | Reinbold, G.W.; LB C | GCA_021133715.1 |
| 96 | LDB | CIRM BIA 1581 | CIRM | Artisanal yoghurt, Crete, Greece | 1987 | Zourari, A.K; ZL031B4 | GCA_021133685.1 |
| 97 | LDB | CIRM BIA 1614 | CIRM | Yoghurt, Île-de-France, France | Unknown | Chevalier, R.; LY3 | GCA_021133675.1 |
| 98 | LDB | CIRM BIA 1623 | CIRM | Yoghurt, the Netherlands | Unknown | NIZO Ib | GCA_021133735.1 |
| 99 | LDB | CIRM BIA 2159 | CIRM | Yoghurt (Bulgarian), Belgium | 1960 | Accolas, J.P.; RM7 | GCA_021133755.1 |
| 100 | LDB | DSM 20081 T | DSMZ | Bulgarian yoghourt (country of origin unknown) | Before 22.08.1990 | Orla-Jensen, S.; 14 | GCA_000056065.1 |
| 101 | LDS | DSM 24966 T | DSMZ | Sunki, Japan | 2004 | K. Watanabe; YIT 11221 | GCA_001888965.1 |
| Minimal Inhibitory Concentrations (mg/L) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotic | 0.016 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 |
| AMP (2) | 20 (16/4/0) | 37 (30/6/1) | 28 (23/3/2) | 2 (1/1/0) | 3 (1/2/0) | 0 | 0 | 0 | 0 | 0 | |||||||
| VAN (2) | 30 (19/10/1) | 47 (40/5/2) | 10 (9/1/0) | 2 (2/0/0) | 1 (1/0/0) | 0 | 0 | 0 | 0 | 0 | |||||||
| GEN (16) | 18 (11/7/0) | 28 (22/3/3) | 30 (26/4/0) | 10 (9/1/0) | 4 (3/1/0) | 0 | 0 | 0 | 0 | 0 | |||||||
| KAN (16) | 5 (0/5/0) | 8 (5/3/0) | 9 (8/1/0) | 26 (21/3/2) | 29 (26/2/1) | 13 (11/2/0) | 0 | 0 | 0 | 0 | |||||||
| STR (16) | 1 (0/1/0) | 5 (2/3/0) | 8 (7/1/0) | 45 (37/5/3) | 23 (17/6/0) | 7 (7/0/0) | 1 (1/0/0) | 0 | 0 | 0 | |||||||
| ERY (1) | 29 (19/9/1) | 37 (33/4/0) | 20 (16/2/2) | 3 (3/0/0) | 0 | 0 | 0 | 1 (0/1/0) | 0 | 0 | |||||||
| CLI (4) | 70 (57/11/2) | 17 (13/4/0) | 1 (1/0/0) | 0 | 2 (0/1/1) | 0 | 0 | 0 | 0 | 0 | |||||||
| TET (4) | 1 (0/1/0) | 1 (1/0/0) | 11 (4/7/0) | 19 (15/4/0) | 21 (19/1/1) | 33 (28/3/2) | 4 (4/0/0) | 0 | 0 | 0 | |||||||
| CHL (4) | 2 (2/0/0) | 1 (1/0/0) | 2 (2/0/0) | 4 (3/1/0) | 35 (26/8/1) | 38 (30/6/2) | 8 (7/1/0) | 0 | 0 | 0 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shani, N.; Oberhaensli, S.; Berthoud, H.; Schmidt, R.S.; Bachmann, H.-P. Antimicrobial Susceptibility of Lactobacillus delbrueckii subsp. lactis from Milk Products and Other Habitats. Foods 2021, 10, 3145. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10123145
Shani N, Oberhaensli S, Berthoud H, Schmidt RS, Bachmann H-P. Antimicrobial Susceptibility of Lactobacillus delbrueckii subsp. lactis from Milk Products and Other Habitats. Foods. 2021; 10(12):3145. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10123145
Chicago/Turabian StyleShani, Noam, Simone Oberhaensli, Hélène Berthoud, Remo S. Schmidt, and Hans-Peter Bachmann. 2021. "Antimicrobial Susceptibility of Lactobacillus delbrueckii subsp. lactis from Milk Products and Other Habitats" Foods 10, no. 12: 3145. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10123145