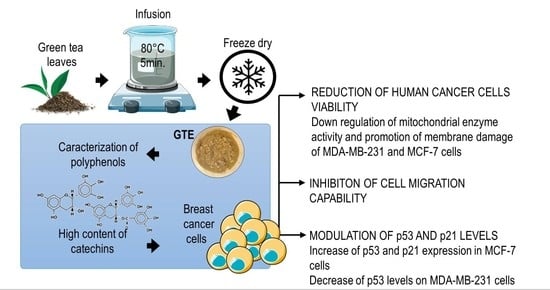
Green Tea (Camellia sinensis) Extract Induces p53-Mediated Cytotoxicity and Inhibits Migration of Breast Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Green Tea Extract Polyphenols Quantification and Antioxidant Capacity
2.3. Characterization of Green Tea Extract Polyphenols by HPLC
2.4. Cell Culture
2.5. Cell Treatment
2.6. Cell Viability
2.7. Selectivity Index
2.8. Wound-Healing Assay
2.9. Western Blotting
2.10. Immunocytochemistry
2.11. Statistical Analysis
3. Results
3.1. Time and Temperature Did Not Influence Polyphenol Content and Antioxidant Capacity of Green Tea Extract
3.2. Green Tea Extract Reduces MDA-MB-231 and MCF-7 Cells Viability
3.3. Green Tea Extract Inhibits Breast Cancer Cell Migration
3.4. p53 and p21 Were Modulated by Green Tea Extract
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Seow, W.J.; Yanwen, D.L.; Pan, W.; Gunther, S.H.; Sim, X.; Torta, F.; Herr, D.R.; Kovalik, J.; Jianhong, C.; Khoo, C.M.; et al. Coffee, Black Tea, and Green Tea Consumption in Relation to Plasma Metabolites in an Asian Population. Mol. Nutr. Food Res. 2020, 64, 2000527. [Google Scholar] [CrossRef]
- Fang, Z.T.; Song, C.J.; Xu, H.R.; Ye, J.H. Dynamic changes in flavonol glycosides during production of green, yellow, white, oolong and black teas from Camellia sinensis L. (cv. Fudingdabaicha). Int. J. Food Sci. Technol. 2019, 54, 490–498. [Google Scholar] [CrossRef]
- Chang, K. World tea production and trade Current and future development. In Food and Agriculture Organization of the United Nations; FAO: Rome, Italy, 2015. [Google Scholar]
- Graham, H.N. Green Tea Composition, Consumption and Polyphenol Chemistry. Prev. Med. (Baltim.) 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Cheng, W.H. Green Tea: An Ancient Antioxidant Drink for Optimal Health? J. Nutr. 2019, 149, 1877–1879. [Google Scholar] [CrossRef]
- Koch, W. Dietary polyphenols-important non-nutrients in the prevention of chronic noncommunicable diseases. A systematic review. Nutrients 2019, 11, 1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [Green Version]
- Gianfredi, V.; Nucci, D.; Abalsamo, A.; Acito, M.; Villarini, M.; Moretti, M.; Realdon, S. Green tea consumption and risk of breast cancer and recurrence—A systematic review and meta-analysis of observational studies. Nutrients 2018, 10, 1886. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Zhu, L.; Wang, K.; Yan, Y.; He, J.; Ren, Y. Green tea consumption and risk of breast cancer: A systematic review and updated meta-analysis of case-control studies. Medicine (Baltimore) 2019, 98, 27. [Google Scholar] [CrossRef]
- Kim, T.L.; Jeong, G.H.; Yang, J.W.; Lee, K.H.; Kronbichler, A.; Van Der Vliet, H.J.; Grosso, G.; Galvano, F.; Aune, D.; Kim, J.Y.; et al. Tea Consumption and Risk of Cancer: An Umbrella Review and Meta-Analysis of Observational Studies. Adv. Nutr. 2020, 11, 1437–1452. [Google Scholar] [CrossRef]
- Filippini, T.; Malavolti, M.; Borrelli, F.; Izzo, A.A.; Fairweather-Tait, S.J.; Horneber, M.; Vinceti, M. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst. Rev. 2020, 3. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Wang, X. The Metabolic Mechanisms of Breast Cancer Metastasis. Front. Oncol. 2021, 10, 2942. [Google Scholar] [CrossRef] [PubMed]
- Kapinova, A.; Stefanicka, P.; Kubatka, P.; Zubor, P.; Uramova, S.; Kello, M.; Mojzis, J.; Blahutova, D.; Qaradakhi, T.; Zulli, A.; et al. Are plant-based functional foods better choice against cancer than single phytochemicals? A critical review of current breast cancer research. Biomed. Pharmacother. 2017, 96, 1465–1477. [Google Scholar] [CrossRef]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary phytochemicals and cancer chemoprevention: A review of the clinical evidence. Oncotarget 2016, 7, 52517–52529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mocanu, M.M.; Nagy, P.; Szöllosi, J.; Vanden Eynde, J.J.; Mayence, A. Chemoprevention of breast cancer by dietary polyphenols. Molecules 2015, 20, 22578–22620. [Google Scholar] [CrossRef] [Green Version]
- Gan, R.-Y.; Li, H.-B.; Sui, Z.-Q.; Corke, H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Crit. Rev. Food Sci. Nutr. 2018, 58, 924–941. [Google Scholar] [CrossRef]
- Shirakami, Y.; Shimizu, M. Possible mechanisms of green tea and its constituents against cancer. Molecules 2018, 23, 2284. [Google Scholar] [CrossRef] [Green Version]
- Musial, C.; Alicja Kuban-Jankowska, M.G.-P. Beneficial properties of green tea. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogelstein, B.; Lane, D.L.; Levine, A.J. Surfing the p53 network. Nature 2000, 408, 307–310. [Google Scholar] [CrossRef]
- da Costa, D.C.F.; Fialho, E.; Silva, J. Cancer Chemoprevention by Resveratrol: The p53 Tumor Suppressor Protein as a Promising Molecular Target. Molecules 2017, 22, 1014. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.; Reale, M.; Ullah, H.; Sureda, A.; Tejada, S.; Wang, Y.; Zhang, Z.J.; Xiao, J. Anti-cancer effects of polyphenols via targeting p53 signaling pathway: Updates and future directions. Biotechnol. Adv. 2020, 38, 107385. [Google Scholar] [CrossRef] [PubMed]
- Koss-Mikołajczyk, I.; Kusznierewicz, B.; Bartoszek, A. The relationship between phytochemical composition and biological activities of differently pigmented varieties of berry fruits; comparison between embedded in food matrix and isolated anthocyanins. Foods 2019, 8, 646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extracts consumption in adults—Results of a systematic review. Regul. Toxicol. Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef]
- Oketch-Rabah, H.A.; Roe, A.L.; Rider, C.V.; Bonkovsky, H.L.; Giancaspro, G.I.; Navarro, V.; Paine, M.F.; Betz, J.M.; Marles, R.J.; Casper, S.; et al. United States Pharmacopeia (USP) comprehensive review of the hepatotoxicity of green tea extracts. Toxicol. Rep. 2020, 7, 386–402. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, Z.; Han, Y.; Wang, J.; Wang, Y.; Chen, X.; Shao, Y.; Cheng, Y.; Zhou, W.; Lu, X.; et al. A review on anti-cancer effect of green tea catechins. J. Funct. Foods 2020, 74, 104172. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Benzie, I.F.F.; Szeto, Y.T. Total antioxidant capacity of teas by the ferric reducing/ antioxidant power assay. J. Agric. Food Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Kohri, T.; Matsumoto, N.; Yamakawa, M.; Suzuki, M.; Nanjo, F.; Hara, Y.; Oku, N. Metabolic Fate of (−)-[4-3H]Epigallocatechin Gallate in Rats after Oral Administration. J. Agric. Food Chem. 2001, 49, 4102–4112. [Google Scholar] [CrossRef]
- Koch, A.; Tamez, P.; Pezzuto, J.; Soejarto, D. Evaluation of plants used for antimalarial treatment by the Maasai of Kenya. J. Ethnopharmacol. 2005, 101, 95–99. [Google Scholar] [CrossRef]
- Ferretti, G.D.D.S.; da Costa, D.C.F.; Silva, J.L.; Pereira Rangel, L. Methods to screen compounds against mutant p53 misfolding and aggregation for cancer therapeutics. In Protein Misfolding Diseases; Humana Press: New York, NY, USA, 2019; pp. 265–277. ISBN 9781493988204. [Google Scholar]
- Tang, G.Y.; Meng, X.; Gan, R.Y.; Zhao, C.N.; Liu, Q.; Feng, Y.B.; Li, S.; Wei, X.L.; Atanasov, A.G.; Corke, H.; et al. Health functions and related molecular mechanisms of tea components: An update review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. Biology Hallmarks of Cancer. Holland-Frei Cancer Med. 2016, 1–10. [Google Scholar] [CrossRef]
- Donlao, N.; Ogawa, Y. The influence of processing conditions on catechin, caffeine and chlorophyll contents of green tea (Camelia sinensis) leaves and infusions. LWT 2019, 116, 108567. [Google Scholar] [CrossRef]
- Wang, H.; Helliwell, K. Epimerisation of catechins in green tea infusions. Food Chem. 2000, 70, 337–344. [Google Scholar] [CrossRef]
- Saito, S.T.; Fröehlich, P.E.; Gosmann, G.; Bergold, A.M. Full Validation of a Simple Method for Determination of Catechins and Caffeine in Brazilian Green Tea (Camellia sinensis var. assamica) Using HPLC. Chromatographia 2007, 65, 607–610. [Google Scholar] [CrossRef]
- Phan, N.L.-C.; Pham, K.D.; Nguyen, M.T.-T.; Phan, N.K.; Truong, K.D.; Van Pham, P. Anti-tumor activity of plant extracts against human breast cancer cells are different in monolayer and three-dimensional cell culture screening models: A comparison on 34 extracts. Biomed. Res. Ther. 2020, 7, 3667–3677. [Google Scholar] [CrossRef]
- Abdelhamed, S.; Yokoyama, S.; Hafiyani, L.; Kalauni, S.K.; Hayakawa, Y.; Awale, S.; Saiki, I. Identification of plant extracts sensitizing breast cancer cells to TRAIL. Oncol. Rep. 2013, 29, 1991–1998. [Google Scholar] [CrossRef] [Green Version]
- Cross, S.E.; Jin, Y.; Tondre, J.; Leporatti, S.; Vergara, D.; Zacheo, A.; Vergara, D.; Martignago, R.; Leporatti, S.; Cross, S.E.; et al. Green tea extract selectively targets nanomechanics of live metastatic cancer cells. Nanotechnology 2011, 22, 215101. [Google Scholar] [CrossRef]
- Lee, M.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F. Pharmacokinetics of Tea Catechins after Ingestion of Green Tea and (−)-Epigallocatechin-3-gallate by Humans—Cancer Epidemiology, Biomarkers & Prevention. Cancer Epidemiol. 2002, 11, 1025–1032. [Google Scholar]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Seo, E.J.; Wu, C.F.; Ali, Z.; Wang, Y.H.; Khan, S.I.; Walker, L.A.; Khan, I.A.; Efferth, T. Both phenolic and non-phenolic green tea fractions inhibit migration of cancer cells. Front. Pharmacol. 2016, 7, 398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deb, G.; Thakur, V.S.; Limaye, A.M.; Gupta, S. Epigenetic induction of tissue inhibitor of matrix metalloproteinase-3 by green tea polyphenols in breast cancer cells. Mol. Carcinog. 2015, 54, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Thangapazham, R.L.; Passi, N.; Maheshwari, R.K. Green tea polyphenol and epigallocatechin gallate induce apoptosis and inhibit invasion in human breast cancer cells. Cancer Biol. Ther. 2007, 6, 1938–1943. [Google Scholar] [CrossRef] [Green Version]
- Etienne-Selloum, N.; Dandache, I.; Sharif, T.; Auger, C.; Schini-Kerth, V.B. Polyphenolic Compounds Targeting p53-Family Tumor Suppressors: Current Progress and Challenges. Futur. Asp. Tumor Suppressor Gene 2013, 129–167. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Chang, X.; Yan, R.; Rong, C.; Yang, C.; Cheng, S.; Gu, X.; Yao, H.; Hou, X.; Mo, Y.; et al. (−)-Epigallocatechin-3-gallate induces cancer cell apoptosis via acetylation of amyloid precursor protein. Med. Oncol. 2015, 32, 390. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Tong, Q.; Liu, X.; Yu, Z.; Zhang, J.; Gao, B. Epigallocatechin-3-gallate inhibits growth and induces apoptosis in esophageal cancer cells through the demethylation and reactivation of the p16 gene. Oncol. Lett. 2017, 14, 1152–1156. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Yan, J.; Luo, L.; Ma, M.; Zhu, H. Preparation and characterization of (−)-Epigallocatechin-3-gallate (EGCG)-loaded nanoparticles and their inhibitory effects on Human breast cancer MCF-7 cells. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Wade Harper, J.; Adami, G.R.; Wei, N.; Keyomarsi, K.; Elledge, S.J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993, 75, 805–816. [Google Scholar] [CrossRef]
- El-deiry, W.S.; Tokino, T.; Velculescu, V.E.; Levy, D.B.; Parsons, R.; Trent, J.M.; Lin, D.; Mercer, W.E.; Kinzler, K.W.; Vogelstein, B. WAF1, a Potential Mediator of p53 Tumor Suppression. Cell Press 1993, 75, 817–825. [Google Scholar] [CrossRef]
- Silva, J.L.; Gallo, C.V.D.M.; Costa, D.C.F.; Rangel, L.P. Prion-like aggregation of mutant p53 in cancer. Trends Biochem. Sci. 2014, 39, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.A.J.; Vousden, K.H. p53 Mutations in Cancer. Nat. Cell Biol. 2013, 15, 2–8. [Google Scholar] [CrossRef] [PubMed]





| Compound | Content (µg/mL) |
|---|---|
| Catechin (C) | 5.06 ± 0.15 |
| Epicatechin gallate (ECG) | 9.44 ± 0.01 |
| Epigallocatechin (EGC) | 31.64 ± 0.69 |
| Epigallocatechin gallate (EGCG) | 32.83 ± 0.15 |
| ∑ catechins | 78.97 ± 0.44 |
| IC50 (µg/mL) | MCF-7 | MDA-MB-231 |
|---|---|---|
| 24 h | 324 | 133 |
| Selectively index (SI) | 31.1 | 75.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, R.A.; Andrade, E.D.S.; Monteiro, M.; Fialho, E.; Silva, J.L.; Daleprane, J.B.; Ferraz da Costa, D.C. Green Tea (Camellia sinensis) Extract Induces p53-Mediated Cytotoxicity and Inhibits Migration of Breast Cancer Cells. Foods 2021, 10, 3154. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10123154
Santos RA, Andrade EDS, Monteiro M, Fialho E, Silva JL, Daleprane JB, Ferraz da Costa DC. Green Tea (Camellia sinensis) Extract Induces p53-Mediated Cytotoxicity and Inhibits Migration of Breast Cancer Cells. Foods. 2021; 10(12):3154. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10123154
Chicago/Turabian StyleSantos, Ronimara A., Emmanuele D. S. Andrade, Mariana Monteiro, Eliane Fialho, Jerson L. Silva, Julio B. Daleprane, and Danielly C. Ferraz da Costa. 2021. "Green Tea (Camellia sinensis) Extract Induces p53-Mediated Cytotoxicity and Inhibits Migration of Breast Cancer Cells" Foods 10, no. 12: 3154. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10123154