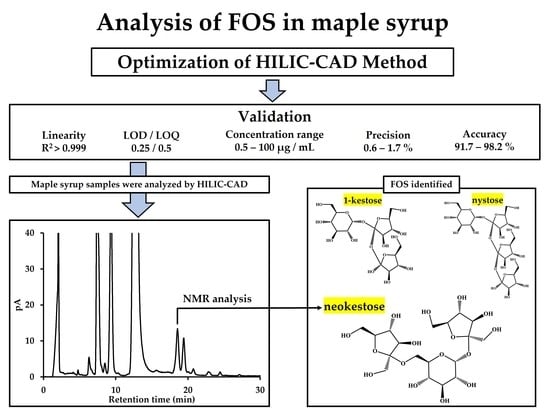
Separation of Fructosyl Oligosaccharides in Maple Syrup by Using Charged Aerosol Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Sample Preparation
2.3. Analysis by High-Performance Liquid Chromatography with CAD (HPLC-CAD)
2.4. HPLC-CAD Method Validation
2.4.1. Linearity
2.4.2. LOD and LOQ
2.4.3. Precision
2.4.4. Accuracy
2.5. Size Exclusion Chromatography
2.6. Nuclear Magnetic Resonance (NMR)
2.7. Statistical Analysis
3. Results
3.1. Developments of the Method for the Separation of FOSs
3.2. Validation of the Optimized Method
3.3. Analysis of Carbohydrates Observed in Maple Syrup
3.4. Structural Analysis of Mapletriose1 by NMR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skinner, C.B.; DeGaetano, A.T.; Chabot, B.F. Implications of twenty-first century climate change on Northeastern United States maple syrup production: Impacts and adaptations. Clim. Chang. 2009, 100, 685–702. [Google Scholar] [CrossRef]
- Nagai, N.; Ito, Y.; Taga, A. Comparison of the enhancement of plasma glucose levels in type 2 diabetes Otsuka Long-Evans Tokushima fatty rats by oral administration of sucrose or maple syrup. J. Oleo Sci. 2013, 62, 737–743. [Google Scholar] [CrossRef]
- Nagai, N.; Yamamoto, T.; Tanabe, W.; Ito, Y.; Kurabuchi, S.; Mitamura, K.; Taga, A. Changes in plasma glucose in Otsuka Long-Evans Tokushima fatty rats after oral administration of maple syrup. J. Oleo Sci. 2015, 64, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Nishita, T.; Taga, A. Dark-colored maple syrup treatment induces S-phase cell cycle arrest via reduced proliferating cell nuclear antigen expression in colorectal cancer cells. Oncol. Lett. 2019, 17, 2713–2720. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sato, K.; Kubota, Y.; Mitamura, K.; Taga, A. Effect of dark-colored maple syrup on cell proliferation of human gastrointestinal cancer cell. Biomed. Rep. 2017, 7, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Uemura, K.; Moriyama, K.; Mitamura, K.; Taga, A. Inhibitory effect of maple syrup on the cell growth and invasion of human colorectal cancer cells. Oncol. Rep. 2015, 33, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Ball, D.W. The chemical composition of maple syrup. J. Chem. Educ. 2007, 84, 1647–1650. [Google Scholar] [CrossRef]
- Perkins, T.D.; van den Berg, A.K. Chapter 4 maple syrup-production, composition, chemistry, and sensory characteristics. Adv. Food Nutr. Res. 2009, 56, 101–143. [Google Scholar]
- Mellado-Mojica, E.; Seeram, N.P.; López, M.G. Comparative analysis of maple syrups and natural sweeteners: Carbohydrates composition and classification (differentiation) by HPAEC-PAD and FTIR spectroscopy-chemometrics. J. Food Compos. Anal. 2016, 52, 1–8. [Google Scholar] [CrossRef]
- Taga, A.; Kodama, S. Analysis of reducing carbohydrates and fructosyl saccharides in maple syrup and maple sugar by CE. Chromatographia 2012, 75, 1009–1016. [Google Scholar] [CrossRef]
- Sun, J.; Ma, H.; Seeram, N.P.; Rowley, D.C. Detection of inulin, a prebiotic polysaccharide, in maple syrup. J. Agric. Food Chem. 2016, 64, 7142–7147. [Google Scholar] [CrossRef]
- Sato, K.; Nagai, N.; Yamamoto, T.; Mitamura, K.; Taga, A. Identification of a novel oligosaccharide in maple syrup as a potential alternative saccharide for diabetes mellitus patients. Int. J. Mol. Sci. 2019, 20, 5041. [Google Scholar] [CrossRef]
- Hidaka, H.; Tashiro, Y.; Eida, T. Proliferation of bifidobacteria by oligosaccharides and their useful effect on human health. Bifid-Microflora 1991, 10, 65–79. [Google Scholar] [CrossRef]
- Molis, C.; Flourie, B.; Ouarne, F.; Gailing, M.F.; Lartigue, S.; Guibert, A.; Bornet, F.; Galmiche, J.P. Digestion, excretion, and energy value of fructooligosaccharides in healthy humans. Am. J. Clin. Nutr. 1996, 64, 324–328. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Zhou, B.; Zhao, J.; Li, S. Determination of fructooligosaccharides in burdock using HPLC and microwave-assisted extraction. J. Agric. Food Chem. 2013, 61, 5888–5892. [Google Scholar] [CrossRef]
- Der Agopian, R.G.; Soares, C.A.; Purgatto, E.; Cordenunsi, B.R.; Lajolo, F.M. Identification of fructooligosaccharides in different banana cultivars. J. Agric. Food Chem. 2008, 56, 3305–3310. [Google Scholar] [CrossRef] [PubMed]
- Chikkerur, J.; Samanta, A.K.; Kolte, A.P.; Dhali, A.; Roy, S. Production of short chain fructo-oligosaccharides from inulin of chicory root using fungal endoinulinase. Appl. Biochem. Biotechnol. 2019, 191, 695–715. [Google Scholar] [CrossRef]
- Zeaiter, Z.; Regonesi, M.E.; Cavini, S.; Labra, M.; Sello, G.; Di Gennaro, P. Extraction and characterization of inulin-type fructans from artichoke wastes and their effect on the growth of intestinal bacteria associated with health. BioMed. Res. Int. 2019, 2019, 1083952. [Google Scholar] [CrossRef] [PubMed]
- Kosasih, W.; Pudjiraharti, S.; Ratnaningrum, D.; Priatni, S. Preparation of inulin from Dahlia Tubers. Procedia Chem. 2015, 16, 190–194. [Google Scholar] [CrossRef]
- Agblevor, F.A.; Hames, B.R.; Schell, D.; Chum, H.L. Analysis of biomass sugars using a novel HPLC method. Appl. Biochem. Biotechnol. 2007, 136, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Farine, S.; Versluis, C.; Bonnici, P.; Heck, A.; Peschet, J.; Puigserver, A.; Biagini, A. Separation and identification of enzymatic sucrose hydrolysis products by high-performance anion-exchange chromatography with pulsed amperometric detection. J. Chromatogr. A 2001, 920, 299–308. [Google Scholar] [CrossRef]
- Hadjikinova, R.; Petkova, N.; Hadjikinov, D.; Denev, P.; Hrusavov, D. Development and validation of HPLC-RID method for determination of sugars and polyols. J. Pharm. Sci. Res. 2017, 9, 1263–1269. [Google Scholar]
- Pitsch, J.; Weghuber, J. Hydrophilic interaction chromatography coupled with charged aerosol detection for simultaneous quantitation of carbohydrates, polyols and ions in food and beverages. Molecules 2019, 24, 4333. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.; Lawton, L.A. Assessment of microcystin purity using charged aerosol detection. J. Chromatogr. A 2010, 1217, 5233–5238. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.A. The analysis of lipids via HPLC with a charged aerosol detector. Lipids 2006, 41, 727–734. [Google Scholar] [CrossRef]
- Socia, A.; Foley, J.P. Direct determination of amino acids by hydrophilic interaction liquid chromatography with charged aerosol detection. J. Chromatogr. A 2016, 1446, 41–49. [Google Scholar] [CrossRef]
- Fukushima, K.; Hashiguchi, K.; Suzuki, T.; Okawara, M.; Sekiguchi, Y. Recent developments in charged aerosol detection technology and applications. Chromatogr. J. Sep. Detect. Sci. 2011, 32, 161–170. [Google Scholar]
- Haq, S.; Adams, G.A. Oligosaccharides from the sap of sugar maple (Acer saccharum Marsh). Can. J. Chem. 1961, 39, 1165–1170. [Google Scholar] [CrossRef]
- ICH Expert Working Group ICH Topic Q 2 (R1) Validation of Analytical Procedures: Text and Methodology Step 5 Note for Guidance on Validation of Analytical Procedures: Text and Methodology (cpmp/ich/381/95) Approval by Cpmp November 1994 Date for Coming into Operation. 1995. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-2-r1-validation-analytical-procedures-text-methodology-step-5_en.pdf (accessed on 7 August 2021).
- Fukushi, E.; Onodera, S.; Yamamori, A.; Shiomi, N.; Kawabata, J. NMR analysis of tri- and tetrasaccharides from asparagus. Magn. Reson. Chem. 2000, 38, 1005–1011. [Google Scholar]
- Eggleston, G.; Wartelle, L.; Cyr, E.S. Detecting adulterated commercial sweet sorghum syrups with ion chromatography oligosaccharide fingerprint profiles. Separations 2016, 3, 20. [Google Scholar] [CrossRef]
- Carabetta, S.; Di Sanzo, R.; Campone, L.; Fuda, S.; Rastrelli, L.; Russo, M. High-performance anion exchange chromatography with Pulsed Amperometric Detection (HPAEC–PAD) and chemometrics for geographical and floral authentication of honeys from Southern Italy (Calabria region). Foods 2020, 9, 1625. [Google Scholar] [CrossRef]
- Corradini, C.; Cavazza, A.; Bignardi, C. High-performance anion-exchange chromatography coupled with Pulsed Electrochemical Detection as a powerful tool to evaluate carbohydrates of food interest: Principles and applications. Int. J. Carbohydr. Chem. 2012, 2012, 487564. [Google Scholar] [CrossRef]
- Homann, A.; Biedendieck, R.; Götze, S.; Jahn, D.; Seibel, J. Insights into polymer versus oligosaccharide synthesis: Mutagenesis and mechanistic studies of a novel levansucrase from Bacillus megaterium. Biochem. J. 2007, 407, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Raga-Carbajal, E.; Munguia, A.L.; Alvarez, L.; Olvera, C. Understanding the transfer reaction network behind the non-processive synthesis of low molecular weight levan catalyzed by Bacillus subtilis levansucrase. Sci. Rep. 2018, 8, 15035. [Google Scholar] [CrossRef] [PubMed]




| Saccharides | Fructose | Glucose | Sucrose | 1-Kestose | Nystose | Fructofuranosyl Nystose | |
|---|---|---|---|---|---|---|---|
| Concentration range (µg/mL) | 0.5–100 | 0.5−100 | 0.5−100 | 0.5−100 | 0.5−100 | 0.5−100 | |
| Linear equation | y = −0.0003x2 + 0.1193x + 0.0279 | y = −0.0003x2 + 0.1268x + 0.0577 | y = −0.0003x2 + 0.1237x + 0.0781 | y = −0.0003x2 + 0.1149x + 0.0539 | y = −0.0002x2 + 0.0980x + 0.0329 | y = −0.0002x2 + 0.0995x + 0.0241 | |
| R2 | 0.9998 | 0.9998 | 0.9996 | 0.9998 | 0.9997 | 0.9999 | |
| LOD/LOQ (µg/mL) | 0.25/0.5 | 0.25/0.5 | 0.25/0.5 | 0.25/0.5 | 0.25/0.5 | 0.25/0.5 | |
| Precision (%) | Intra-day (n = 9) | 0.733 | 0.768 | 0.639 | 0.668 | 0.848 | 0.840 |
| Inter-day (n = 3) | 0.630 | 1.46 | 1.02 | 1.67 | 1.46 | 1.71 | |
| Accuracy (%) | 96.5 | 91.7 | 94.3 | 92.3 | 98.2 | 97.9 | |
| Variables | Golden | Amber | Dark | Very Dark |
|---|---|---|---|---|
| Fructose (µg/10 mg) | 21.3 ± 0.146 | 41.7 ± 0.295 | 65.6 ± 0.442 | 163 ± 1.46 |
| Glucose (µg/10 mg) | 31.6 ± 0.152 | 55.1 ± 0.396 | 83.3 ± 0.464 | 198 ± 2.12 |
| Sucrose (mg/10 mg) | 5.98 ± 0.159 | 5.94 ± 0.138 | 6.04 ± 0.115 | 5.49 ± 0.0506 |
| Mapletriose1 (*) (µg/10 mg) | 20.6 ± 1.23 | 19.6 ± 0.474 | 31.2 ± 0.491 | 40.6 ± 0.573 |
| 1-Kestose (µg/10 mg) | 5.05 ± 0.357 | 10.1 ± 0.383 | 13.6 ± 0.142 | 25.3 ± 0.510 |
| Nystose (µg/10 mg) | 0.244 ± 0.0155 | 0.593 ± 0.00395 | 1.45 ± 0.0191 | 3.08 ± 0.0483 |
| Chemical Shift | |||
|---|---|---|---|
| Residue | Position | Observed (200 MHz) in D2O δC | Observed (800 MHz) in D2O δH |
| Fruf β | 1 | 64.15 | 3.64 (d, 12.6) |
| 3.656 (d, 12.6) | |||
| 2 | 106.42 * | - | |
| 3 | 78.92 | 4.21 (d, 8.9) | |
| 4 | 76.64 | 4.05 (dd-like, ca. 8.9, ca. 8.5) | |
| 5 | 84.07 | 3.88 (ddd, 8.5, 7.3, 3.0) | |
| 6 | 65.14 | 3.78 (dd-like, ca. 12.4, ca. 7.3) | |
| 3.83 (dd-like, ca. 12.4, 3.0) | |||
| Glcp α | 1′ | 94.72 | 5.39 (d, 3.9) |
| 2′ | 73.73 | 3.55 (dd, 9.9, 3.9) | |
| 3′ | 75.15 | 3.736 (dd-like, ca. 9.9, ca. 9.5) | |
| 4′ | 71.89 | 3.51 (dd-like, ca. 10.3, ca. 9.5) | |
| 5′ | 74.25 | 3.93 (ddd-like, ca. 10.3, 4.1, 2.1) | |
| 6′ | 63.02 | 3.78 (dd-like, ca. 11.5, ca. 4.1) | |
| 3.92 (dd-like, ca. 11.5, 2.1) | |||
| Fruf β | 1″ | 62.90 | 3.660 (d, 12.1) |
| 3.744 (d, 12.1) | |||
| 2″ | 106.43 * | - | |
| 3″ | 79.48 | 4.18 (d, 8.7) | |
| 4″ | 77.04 | 4.13 (dd-like, ca. 8.7, ca. 7.8) | |
| 5″ | 83.86 | 3.86 (ddd, 7.8, 6.6, 3.0) | |
| 6″ | 65.07 | 3.69 (dd, 12.4, 6.6) | |
| 3.82 (dd-like, ca. 12.4, 3.0) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, K.; Yamamoto, T.; Mitamura, K.; Taga, A. Separation of Fructosyl Oligosaccharides in Maple Syrup by Using Charged Aerosol Detection. Foods 2021, 10, 3160. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10123160
Sato K, Yamamoto T, Mitamura K, Taga A. Separation of Fructosyl Oligosaccharides in Maple Syrup by Using Charged Aerosol Detection. Foods. 2021; 10(12):3160. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10123160
Chicago/Turabian StyleSato, Kanta, Tetsushi Yamamoto, Kuniko Mitamura, and Atsushi Taga. 2021. "Separation of Fructosyl Oligosaccharides in Maple Syrup by Using Charged Aerosol Detection" Foods 10, no. 12: 3160. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10123160