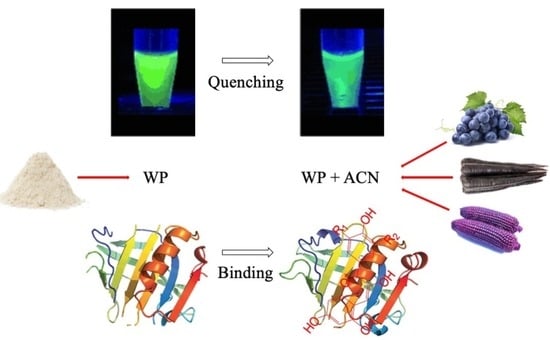
Monitoring the Interaction between Thermally Induced Whey Protein and Anthocyanin by Fluorescence Quenching Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Buffer System and Sample Preparation
2.3. Anthocyanin Identification
2.4. Fluorescence Spectroscopy
2.5. Fluorescence Quenching Analysis
2.6. Thermodynamic Analysis
2.7. Statistical Analysis
3. Results
3.1. Analysis of Fluorescence Spectra of Whey Protein in the Presence of Anthocyanins
3.2. Fluorescence Quenching Study of Anthocyanin-Whey Protein Interaction
3.3. Thermodynamic Analysis between Whey Protein and Anthocyanin
3.4. The Effect of Preheating Temperature on the Fluorescence Spectra of Whey Protein in the Presence of Anthocyanins
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural colorants: Food colorants from natural sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Giusti, M.M. Anthocyanins—nature’s bold, beautiful, and health-promoting colors. Foods 2019, 8, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, U.; Bae, W.J.; Kim, S.J.; Yoon, B.I.; Hong, S.H.; Lee, J.Y.; Hwang, T.K.; Hwang, S.Y.; Wang, Z.; Kim, S.W. Anthocyanin induces apoptosis of DU-145 cells in vitro and inhibits xenograft growth of prostate cancer. Yonsei Med. J. 2015, 56, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torskangerpoll, K.; Andersen, M. Colour stability of anthocyanins in aqueous solutions at various pH values. Food Chem. 2005, 89, 427–440. [Google Scholar] [CrossRef]
- Xiong, S.; Melton, L.D.; Easteal, A.J.; Siew, D. Stability and antioxidant activity of black currant anthocyanins in solution and encapsulated in glucan gel. J. Agric. Food Chem. 2006, 54, 6201–6208. [Google Scholar] [CrossRef]
- He, W.; Mu, H.; Liu, Z.; Lu, M.; Hang, F.; Chen, J.; Chen, J.; Zeng, M.; Qin, F.; He, Z. Effect of preheat treatment of milk proteins on their interactions with cyanidin-3-O-glucoside. Food Res. Int. 2018, 107, 394–405. [Google Scholar] [CrossRef]
- Shpigelman, A.; Israeli, G.; Livney, Y.D. Thermally-induced protein–polyphenol co-assemblies: Beta lactoglobulin-based nanocomplexes as protective nanovehicles for EGCG. Food Hydrocoll. 2010, 24, 735–743. [Google Scholar] [CrossRef]
- Havea, P.; Carr, A.J.; Creamer, L.K. The roles of disulphide and non-covalent bonding in the functional properties of heat-induced whey protein gels. J. Dairy Res. 2004, 71, 330–339. [Google Scholar] [CrossRef]
- He, Z.Y.; Chen, J.; Moser, S.E. Interaction of b-lactoglobulin with (-)-epigallocatechin-3-gallate under different processing conditions of pH and temperature by the fluorescence quenching method. Eur. Food Res. Technol. 2015, 241, 357–366. [Google Scholar] [CrossRef]
- Liang, L.; Subirade, M. Study of the acid and thermal stability of β-lactoglobulin–ligand complexes using fluorescence quenching. Food Chem. 2012, 132, 2023–2029. [Google Scholar] [CrossRef]
- Zorilla, R.; Liang, L.; Remondetto, G.; Subirade, M. Interaction of epigallocatechin-3-gallate with β-lactoglobulin: Molecular characterization and biological implication. Dairy Sci. Technol. 2011, 91, 629. [Google Scholar] [CrossRef] [Green Version]
- Deaville, E.R.; Green, R.J.; Mueller-Harvey, I.; Willoughby, I.; Frazier, R.A. Hydrolyzable tannin structures influence relative globular and random coil protein binding strengths. J. Agric. Food Chem. 2007, 55, 4554–4561. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, P.; Ghosh, A.K.; Ghosh, C. Recent developments on polyphenol–protein interactions: Effects on tea and coffee taste, antioxidant properties and the digestive system. Food Funct. 2012, 3, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, C.D.; Hasni, I.; Bourassa, P.; Tarantilis, P.A.; Polissiou, M.G.; Tajmir-Riahi, H.A. Milk β-lactoglobulin complexes with tea polyphenols. Food Chem. 2011, 127, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Green, R.J.; Frazier, R.A. Interaction of flavonoids with bovine serum albumin: A fluorescence quenching study. J. Agric. Food Chem. 2005, 53, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Maya, I.J.; Campos-Terán, J.; Hernández-Arana, A.; McClements, D.J. Characterization of flavonoid-protein interactions using fluorescence spectroscopy: Binding of pelargonidin to dairy proteins. Food Chem. 2016, 213, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Rojanasasithara, T.; Mutilangi, W.; McClements, D.J. Enhanced stability of anthocyanin-based color in model beverage systems through whey protein isolate complexation. Food Res. Int. 2015, 76, 761–768. [Google Scholar] [CrossRef]
- Stănciuc, N.; Turturică, M.; Oancea, A.M.; Barbu, V.; Ioniţă, E.; Aprodu, I.; Râpeanu, G. Microencapsulation of anthocyanins from grape skins by whey protein isolates and different polymers. Food Bioprocess Technol. 2017, 10, 1715–1726. [Google Scholar] [CrossRef]
- Dawson, R.M.C.; Elliot, D.C.; Elliot, W.H.; Jones, K.M. Data for Biochemical Research, 3rd ed.; Oxford Science Publ., OUP: Oxford, UK, 1986. [Google Scholar]
- Rodriguez-Saona, L.E.; Wrolstad, R.E. Extraction, Isolation, and Purification of Anthocyanins. Curr. Protoc. Food Anal. Chem. 2001, F1.1.1–F1.1.11. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, Q. Effects of thermal denaturation on binding between bixin and whey protein. J. Agric. Food Chem. 2012, 60, 7526–7531. [Google Scholar] [CrossRef] [PubMed]
- Lissi, E.; Abuin, E. On the evaluation of the number of binding sites in proteins from steady state fluorescence measurements. J. Fluoresc. 2011, 21, 1831–1833. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zuo, H.; Shu, L. Comparison of the interaction between three anthocyanins and human serum albumins by spectroscopy. J. Lumin. 2014, 153, 54–63. [Google Scholar] [CrossRef]
- Mehranfar, F.; Bordbar, A.K.; Parastar, H. A combined spectroscopic, molecular docking and molecular dynamic simulation study on the interaction of quercetin with β-casein nanoparticles. J. Photochem. Photobiol. B Biol. 2013, 127, 100–107. [Google Scholar] [CrossRef]
- Miyagusuku-Cruzado, G.; Giusti, M.M. Whey protein addition and its increased light absorption and tinctorial strength of model solutions colored with anthocyanins. J. Dairy Sci. 2020, in press. [Google Scholar]
- Bourassa, P.; Bariyanga, J.; Tajmir-Riahi, H.A. Binding sites of resveratrol, genistein, and curcumin with milk α-and β-caseins. J. Phys. Chem. B 2013, 117, 1287–1295. [Google Scholar] [CrossRef]
- He, Z.; Zhu, H.; Xu, M.; Zeng, M.; Qin, F.; Chen, J. Complexation of bovine β-lactoglobulin with malvidin-3-O-glucoside and its effect on the stability of grape skin anthocyanin extracts. Food Chem. 2016, 209, 234–240. [Google Scholar] [CrossRef]
- Lakowicz, J.R. (Ed.) Principles of Fluorescence Spectroscopy; Springer Science Business Media: Boston, MA, USA, 2013. [Google Scholar]
- Acharya, D.P.; Sanguansri, L.; Augustin, M.A. Binding of resveratrol with sodium caseinate in aqueous solutions. Food Chem. 2013, 141, 1050–1054. [Google Scholar] [CrossRef]
- Tang, J.; Luan, F.; Chen, X. Binding analysis of glycyrrhetinic acid to human serum albumin: Fluorescence spectroscopy, FTIR, and molecular modeling. Bioorgan. Med. Chem. 2006, 14, 3210–3217. [Google Scholar] [CrossRef]
- Faridbod, F.; Ganjali, M.R.; Larijani, B.; Riahi, S.; Saboury, A.A.; Hosseini, M.; Norouzi, P.; Pillip, C. Interaction study of pioglitazone with albumin by fluorescence spectroscopy and molecular docking. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 96–101. [Google Scholar] [CrossRef]
- Khalifa, I.; Nie, R.; Ge, Z.; Li, K.; Li, C. Understanding the shielding effects of whey protein on mulberry anthocyanins: Insights from multispectral and molecular modelling investigations. Int. J. Biol. Macromol. 2018, 119, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Rawel, H.M.; Meidtner, K.; Kroll, J. Binding of selected phenolic compounds to proteins. J. Agric. Food Chem. 2005, 53, 4228–4235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ma, Y. Mechanistic and conformational studies on the interaction of food dye amaranth with human serum albumin by multispectroscopic methods. Food Chem. 2013, 136, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.; Mateus, N.; Pianet, I.; Laguerre, M.; de Freitas, V. Mechanisms of tannin-induced trypsin inhibition: A molecular approach. Langmuir 2011, 27, 13122–13129. [Google Scholar] [CrossRef]
- Palazolo, G.; Rodríguez, F.; Farruggia, B.; Picó, G.; Delorenzi, N. Heat treatment of β-lactoglobulin: Structural changes studied by partitioning and fluorescence. J. Agric. Food Chem. 2000, 48, 3817–3822. [Google Scholar] [CrossRef]
- Stănciuc, N.; Aprodu, I.; Râpeanu, G.; Bahrim, G. Fluorescence spectroscopy and molecular modeling investigations on the thermally induced structural changes of bovine β-lactoglobulin. Innov. Food Sci. Emerg. Technol. 2012, 15, 50–56. [Google Scholar] [CrossRef]




| Parameters | T (°C) | |||
|---|---|---|---|---|
| 25 | 35 | 45 | ||
| Purple corn | Ksv (104 M−1) | 3.02 0.07 | 2.97 0.13 | 3.1 0.05 |
| Kq (1012 M−1s−1) | 3.02 0.07 | 2.97 0.13 | 3.1 0.05 | |
| Ks (103 M−1) | 4.82 0.15 | 4.94 0.28 | 5.70 0.19 | |
| Ho (103 J mol−1) | 6.52 | |||
| Go (104 J mol−1) | −2.10 | −2.18 | −2.29 | |
| So (J mol−1 K−1) | 92.40 | 70.71 | 71.89 | |
| Grape | Ksv (104 M−1) | 2.31 0.07 | 2.33 0.04 | 2.35 0.07 |
| Kq (1012 M−1s−1) | 2.31 0.07 | 2.33 0.04 | 2.35 0.07 | |
| Ks (103 M−1) | 4.09 0.78 | 4.86 | 5.99 1.23 | |
| Ho (103 J mol−1) | −6.49 | |||
| Go (104 J mol−1) | −2.06 | −2.10 | −2.15 | |
| So (J mol−1 K−1) | 47.36 | 68.29 | 67.77 | |
| Black carrot | Ksv (104 M−1) | 2.44 | 2.46 0.04 | 2.52 0.03 |
| Kq (1012 M−1s−1) | 2.44 | 2.46 0.04 | 2.52 0.03 | |
| Ks (103 M−1) | 1.00 0.21 | 1.03 0.12 | 1.14 0.18 | |
| Ho (103 J mol−1) | 4.90 | |||
| Go (104 J mol−1) | −1.71 | −1.78 | −1.86 | |
| So (J mol−1 K−1) | 73.89 | 57.64 | 58.49 | |
| Parameters | Preheat T (°C) | ||||||
|---|---|---|---|---|---|---|---|
| Native | 40 | 50 | 60 | 70 | 80 | ||
| Purple corn | Ksv (104 M−1) | 1.67 0.09 | 1.69 0.02 | 1.71 0.09 | 1.82 0.04 | 1.86 0.07 | 1.98 0.06 |
| Kq (1012 M−1s−1) | 1.67 0.09 | 1.69 0.02 | 1.71 0.09 | 1.82 0.04 | 1.86 0.07 | 1.98 0.06 | |
| Ks (102 M−1) | 3.33 0.93 | 4.78 0.95 | 4.75 0.33 | 4.65 0.71 | 4.08 0.40 | 3.77 0.75 | |
| Grape | Ksv (104 M−1) | 1.44 0.17 | 1.28 0.09 | 1.29 0.06 | 1.31 0.07 | 1.60 0.06 | 1.61 0.03 |
| Kq (1012 M−1s−1) | 1.44 0.17 | 1.28 0.09 | 1.29 0.06 | 1.31 0.07 | 1.60 0.06 | 1.61 0.03 | |
| Ks (102 M−1) | 0.89 0.26 | 1.32 0.10 | 1.31 0.03 | 1.25 0.07 | 1.08 0.53 | 0.97 0.17 | |
| Black carrot | Ksv (103 M−1) | 6.7 0.49 | 6.7 0.57 | 6.7 0.78 | 6.8 0.50 | 7.8 0.99 | 8.8 0.71 |
| Kq (1011 M−1s−1) | 6.7 0.49 | 6.7 0.57 | 6.7 0.78 | 6.8 0.50 | 7.8 0.99 | 8.8 0.71 | |
| Ks (102 M−1) | 1.07 0.49 | 2.66 0.23 | 2.54 0.84 | 2.00 0.15 | 1.42 0.72 | 1.25 0.77 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, S.; Giusti, M.M. Monitoring the Interaction between Thermally Induced Whey Protein and Anthocyanin by Fluorescence Quenching Spectroscopy. Foods 2021, 10, 310. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10020310
Ren S, Giusti MM. Monitoring the Interaction between Thermally Induced Whey Protein and Anthocyanin by Fluorescence Quenching Spectroscopy. Foods. 2021; 10(2):310. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10020310
Chicago/Turabian StyleRen, Shuai, and M. Monica Giusti. 2021. "Monitoring the Interaction between Thermally Induced Whey Protein and Anthocyanin by Fluorescence Quenching Spectroscopy" Foods 10, no. 2: 310. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10020310