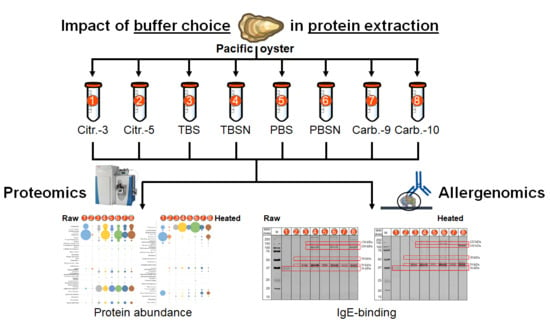
Effects of Extraction Buffer on the Solubility and Immunoreactivity of the Pacific Oyster Allergens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Extraction Buffers
2.2. Preparation of Oyster Soluble Protein Extracts
2.3. Quantification of Protein Content
2.4. Proteomic Profiling of Oyster Extracts
2.5. Mass-Spectrometry Analysis and Protein Identification
2.6. SDS–PAGE and IgE-Reactive Analysis of Oyster Extracts
2.7. Statistical Analysis and Experimental Design
3. Results
3.1. Effects of Extraction Buffers on Soluble Protein Content
3.2. Proteomic Analysis of the Extracts
3.3. Protein Profiling by SDS-PAGE
3.4. Effect of Extraction Buffers on the Presence of IgE-Binding Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wai, C.Y.; Leung, N.Y.; Chu, K.H.; Leung, P.S.; Leung, A.S.; Wong, G.W.; Leung, T.F. Overcoming Shellfish Allergy: How Far Have We Come? Int. J. Mol. Sci. 2020, 21, 2234. [Google Scholar] [CrossRef] [Green Version]
- Ruethers, T.; Taki, A.C.; Johnston, E.B.; Nugraha, R.; Le, T.T.; Kalic, T.; McLean, T.R.; Kamath, S.D.; Lopata, A.L. Seafood allergy: A comprehensive review of fish and shellfish allergens. Mol. Immunol. 2018, 100, 28–57. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Pomés, A.; Davies, J.M.; Gadermaier, G.; Hilger, C.; Holzhauser, T.; Lidholm, J.; Lopata, A.L.; Mueller, G.A.; Nandy, A.; Radauer, C.; et al. WHO/IUIS Allergen Nomenclature: Providing a common language. Mol. Immunol. 2018, 100, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Nugraha, R.; Kamath, S.D.; Johnston, E.; Zenger, K.R.; Rolland, J.M.; O’Hehir, R.E.; Lopata, A.L. Rapid and comprehensive discovery of unreported shellfish allergens using large-scale transcriptomic and proteomic resources. J. Allergy Clin. Immunol. 2018, 141, 1501–1504.e8. [Google Scholar] [CrossRef] [Green Version]
- Cardona, E.E.G.; Heathcote, K.; Teran, L.M.; Righetti, P.G.; Boschetti, E.; D’Amato, A. Novel low-abundance allergens from mango via combinatorial peptide libraries treatment: A proteomics study. Food Chem. 2018, 269, 652–660. [Google Scholar] [CrossRef]
- Suzuki, M.; Kobayashi, Y.; Hiraki, Y.; Nakata, H.; Shiomi, K. Paramyosin of the disc abalone Haliotis discus discus: Identification as a new allergen and cross-reactivity with tropomyosin. Food Chem. 2011, 124, 921–926. [Google Scholar] [CrossRef]
- Poms, R.E.; Capelletti, C.; Anklam, E. Effect of roasting history and buffer composition on peanut protein extraction efficiency. Mol. Nutr. Food Res. 2004, 48, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, J.; Ansari, P.; Kern, C.; Ludwig, T.; Baumgartner, S. Effects of different extraction buffers on peanut protein detectability and lateral flow device (LFD) performance. Food Addit. Contam. Part A 2012, 29, 1–11. [Google Scholar] [CrossRef]
- Lasekan, A.O.; Nayak, B. Effects of buffer additives and thermal processing methods on the solubility of shrimp (Penaeus monodon) proteins and the immunoreactivity of its major allergen. Food Chem. 2016, 200, 146–153. [Google Scholar] [CrossRef] [Green Version]
- Ruethers, T.; Taki, A.C.; Nugraha, R.; Cao, T.T.; Koeberl, M.; Kamath, S.D.; Williamson, N.A.; O’Callaghan, S.; Nie, S.; Mehr, S.S.; et al. Variability of allergens in commercial fish extracts for skin prick testing. Allergy 2019, 74, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Kessler, B.M. Gel-aided sample preparation (GASP)—A simplified method for gel-assisted proteomic sample generation from protein extracts and intact cells. Proteomics 2015, 15, 1224–1229. [Google Scholar] [CrossRef] [Green Version]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef]
- Schwanhäusser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global quantification of mammalian gene expression control. Nat. Cell Biol. 2011, 473, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Ruethers, T.; Taki, A.C.; Karnaneedi, S.; Nie, S.; Kalic, T.; Dai, D.; Daduang, S.; Leeming, M.; Williamson, N.A.; Breiteneder, H.; et al. Expanding the allergen repertoire of salmon and catfish. Allergy 2020. [Google Scholar] [CrossRef]
- Kamath, S.D.; Scheiblhofer, S.; Johnson, C.M.; Machado, Y.; McLean, T.; Taki, A.C.; Ramsland, P.A.; Iyer, S.; Joubert, I.; Hofer, H.; et al. Effect of structural stability on endolysosomal degradation and T-cell reactivity of major shrimp allergen tropomyosin. Allergy 2020, 75, 2909–2919. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Fornecker, L.; Dorsselaer, A.; Carapito, C.; Cianférani, S. Benchmarking sample preparation/digestion protocols reveals tube-gel being a fast and repeatable method for quantitative proteomics. Proteom. 2016, 16, 2953–2961. [Google Scholar] [CrossRef]
- Rolland, J.M.; Varese, N.P.; Abramovitch, J.B.; Anania, J.; Nugraha, R.; Kamath, S.; Hazard, A.; Lopata, A.L.; O’Hehir, R.E. Effect of Heat Processing on IgE Reactivity and Cross-Reactivity of Tropomyosin and Other Allergens of Asia-Pacific Mollusc Species: Identification of Novel Sydney Rock Oyster Tropomyosin Sac g 1. Mol. Nutr. Food Res. 2018, 62, e1800148. [Google Scholar] [CrossRef] [Green Version]
- Martins, L.M.L.; Peltre, G.; da Costa Faro, C.J.F.; Pires, E.M.V.V.; da Cruz Inácio, F.F. The Helix aspersa (Brown Garden Snail) Allergen Repertoire. Int. Arch. Allergy Immunol. 2005, 136, 7–15. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.-X.; Liu, M.; Maleki, S.J.; Zhang, M.-L.; Liu, Q.-M.; Cao, M.-J.; Su, W.-J.; Liu, G.-M. Triosephosphate Isomerase and Filamin C Share Common Epitopes as Novel Allergens of Procambarus clarkii. J. Agric. Food Chem. 2017, 65, 950–963. [Google Scholar] [CrossRef]
- Kalyanasundaram, A.; Santiago, T.C. Identification and characterization of new allergen troponin C (Pen m 6.0101) from Indian black tiger shrimp Penaeus monodon. Eur. Food Res. Technol. 2015, 240, 509–515. [Google Scholar] [CrossRef]
- L’Hocine, L.; Pitre, M. Quantitative and qualitative optimization of allergen extraction from peanut and selected tree nuts. Part 2. Optimization of buffer and ionic strength using a full factorial experimental design. Food Chem. 2016, 194, 820–827. [Google Scholar] [CrossRef]
- Peiren, N.; Vanrobaeys, F.; De Graaf, D.C.; Devreese, B.; Van Beeumen, J.; Jacobs, F.J. The protein composition of honeybee venom reconsidered by a proteomic approach. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2005, 1752, 1–5. [Google Scholar] [CrossRef]
- Van Vaerenbergh, M.; Debyser, G.; Devreese, B.; De Graaf, D.C. Exploring the hidden honeybee (Apis mellifera) venom proteome by integrating a combinatorial peptide ligand library approach with FTMS. J. Proteom. 2014, 99, 169–178. [Google Scholar] [CrossRef]
- L’Hocine, L.; Pitre, M. Quantitative and qualitative optimization of allergen extraction from peanut and selected tree nuts. Part 1. Screening of optimal extraction conditions using a D-optimal experimental design. Food Chem. 2016, 194, 780–786. [Google Scholar] [CrossRef]
- Kramer, R.M.; Shende, V.R.; Motl, N.; Pace, C.N.; Scholtz, J.M. Toward a Molecular Understanding of Protein Solubility: Increased Negative Surface Charge Correlates with Increased Solubility. Biophys. J. 2012, 102, 1907–1915. [Google Scholar] [CrossRef] [Green Version]
- Tomé, L.I.N.; Jorge, M.; Gomes, J.R.B.; Coutinho, J.A.P. Toward an Understanding of the Aqueous Solubility of Amino Acids in the Presence of Salts: A Molecular Dynamics Simulation Study. J. Phys. Chem. B 2010, 114, 16450–16459. [Google Scholar] [CrossRef] [Green Version]
- Kamath, S.D.; Rahman, A.M.A.; Komoda, T.; Lopata, A.L. Impact of heat processing on the detection of the major shellfish allergen tropomyosin in crustaceans and molluscs using specific monoclonal antibodies. Food Chem. 2013, 141, 4031–4039. [Google Scholar] [CrossRef]
- Suzuki, M.; Shimizu, K.; Kobayashi, Y.; Ishizaki, S.; Shiomi, K. Paramyosin from the Disc Abalone Haliotis Discus Discus. J. Food Biochem. 2014, 38, 444–451. [Google Scholar] [CrossRef]
- Yu, C.; Gao, X.; Lin, H.; Xu, L.; Ahmed, I.; Khan, M.U.; Xu, M.; Chen, Y.; Li, Z. Purification, Characterization, and Three-Dimensional Structure Prediction of Paramyosin, a Novel Allergen of Rapana venosa. J. Agric. Food Chem. 2020, 68, 14632–14642. [Google Scholar] [CrossRef]
- Lee, C.-S.; Tsai, L.-C.; Chao, P.-L.; Lin, C.-Y.; Hung, M.-W.; Chien, A.-I.; Chiang, Y.-T.; Han, S.-H. Protein sequence analysis of a novel 103-kDa Dermatophagoides pteronyssinus mite allergen and prevalence of serum immunoglobulin E reactivity to rDer p 11 in allergic adult patients. Clin. Exp. Allergy 2004, 34, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, N.E.; Lopata, A.L. Anisakis—A food-borne parasite that triggers allergic host defences. Int. J. Parasitol. 2013, 43, 1047–1057. [Google Scholar] [CrossRef]
- Kantha, S.S.; Watabe, S.; Hashimoto, K. Comparative Biochemistry of Paramyosin? A review. J. Food Biochem. 1990, 14, 61–88. [Google Scholar] [CrossRef]
- Lindman, S.; Xue, W.-F.; Szczepankiewicz, O.; Bauer, M.C.; Nilsson, H.; Linse, S. Salting the Charged Surface: pH and Salt Dependence of Protein G B1 Stability. Biophys. J. 2006, 90, 2911–2921. [Google Scholar] [CrossRef] [Green Version]
- Miyazawa, H.; Fukamachi, H.; Inagaki, Y.; Reese, G.; Daul, C.B.; Lehrer, S.B.; Inouye, S.; Sakaguchi, M. Identification of the first major allergen of a squid (Todarodes pacificus). J. Allergy Clin. Immunol. 1996, 98, 948–953. [Google Scholar] [CrossRef]
- Ishikawa, M.; Ishida, M.; Shimakura, K.; Nagashima, Y.; Shiomi, K. Purification and IgE-binding epitopes of a major allergen in the gastropod Turbo cornutus. Biosci. Biotechnol. Biochem. 1998, 62, 337–343. [Google Scholar] [CrossRef]
- Lopata, A.L.; Zinn, C.; Potter, P.C. Characteristics of hypersensitivity reactions and identification of a unique 49 kd IgE-binding protein (Hal-m-1) in abalone (Haliotis midae). J. Allergy Clin. Immunol. 1997, 100, 642–648. [Google Scholar] [CrossRef]
- Kamath, S.D.; Rahman, A.M.A.; Voskamp, A.; Komoda, T.; Rolland, J.M.; O’Hehir, R.E.; Lopata, A.L. Effect of heat processing on antibody reactivity to allergen variants and fragments of black tiger prawn: A comprehensive allergenomic approach. Mol. Nutr. Food Res. 2014, 58, 1144–1155. [Google Scholar] [CrossRef]






| Buffer | pH | Composition |
|---|---|---|
| Citrate-3 | 3.0 | Citric acid 0.082 M Trisodium citrate 0.018 M |
| Citrate-5 | 5.0 | Citric acid 0.065 M Trisodium citrate 0.035 M |
| TBS | 7.4 | Tris 25 mM Potassium Chloride 3.0 mM Sodium Chloride 140 mM |
| TBSN | 7.4 | Tris 25 mM Potassium Chloride 3.0 mM Sodium Chloride 1 M |
| PBS | 7.4 | Phosphate 10 mM Potassium Chloride 2.7 mM Sodium Chloride 137 mM |
| PBSN | 7.4 | Phosphate 10 mM Potassium Chloride 2.7 mM Sodium Chloride 1 M |
| Carbonate-9 | 9.2 | Sodium Carbonate 0.01 M Sodium Bicarbonate 0.09 M |
| Carbonate-10 | 10.3 | Sodium Carbonate 0.07 M Sodium Bicarbonate 0.03 M |
| Sex | Age (yrs) | Total IgE (kU/L) | Specific IgE (ImmunoCAP kU/L) | Skin Prick Test | |||||
|---|---|---|---|---|---|---|---|---|---|
| Oyster (f290) | Shrimp (f24) | HDM (d1) | Shrimp | Oyster | HDM | ||||
| 1 | M | 50 | 976 | 2.04 | 9.03 | 13.60 | NT | NT | 12 mm |
| 2 | F | 28 | 461 | 0.11 | 0.36 | 54.8 | NT | NT | 6 mm |
| 3 | M | 43 | 194 | NT | 1.41 | 0.35 | 10 mm | 3 mm | 10 mm |
| 4 | F | 38 | 28 | 3.75 | 9.82 | 2.66 | NT | NT | 0 mm |
| 5 | M | 38 | 183 | 1.04 | 6.84 | 31.70 | NT | NT | NT |
| Buffer | Protein Concentration (mg/mL) | |
|---|---|---|
| Raw | Heated | |
| Citrate-3 | 2.26 ± 0.29 a | 2.35 ± 0.39 a,b |
| Citrate-5 | 3.04 ± 0.14 a | 2.42 ± 0.09 a |
| TBS | 6.99 ± 0.28 b | 1.61 ± 0.05 c |
| TBSN | 9.08 ± 0.29 c | 1.86 ± 0.05 c,d |
| PBS | 7.69 ± 0.23 b,d | 1.74 ± 0.04 c,e |
| PBSN | 9.70 ± 0.97 b,e | 2.07 ± 0.08 b,d,e,f |
| Carbonate-9 | 8.04 ± 0.16 d | 1.88 ± 0.14 c,f |
| Carbonate-10 | 10.43 ± 0.52 e | 4.29 ± 0.13 g |
| Band No | Protein | Accession ID | Exp MW | Theo MW | Mascot Score | Coverage (%) | Number of Significant Peptides | emPAI |
|---|---|---|---|---|---|---|---|---|
| Raw | ||||||||
| 1 | Tropomyosin | B7XC66_CRAGI | 36 | 33 | 1566 | 62 | 19 | 22.48 |
| Myosin heavy chain | K1RSS3_CRAGI | 230 | 1463 | 20 | 33 | 1.42 | ||
| Filamin | K1PW06_CRAGI | 326 | 553 | 12 | 6 | 0.23 | ||
| 2 | Arginine kinase | K1PLF9_CRAGI | 39 | 40 | 1749 | 72 | 23 | 34.84 |
| Tropomyosin | B7XC66_CRAGI | 33 | 1601 | 55 | 19 | 20.33 | ||
| Filamin | K1PW06_CRAGI | 326 | 2533 | 32 | 65 | 1.17 | ||
| 3 | Retinal dehydrogenase I | K1QVG5_CRAGI | 50 | 53 | 1256 | 59 | 43 | 6.72 |
| Aldehyde dehydrogenase | K1QNT7_CRAGI | 58 | 648 | 42 | 17 | 3.01 | ||
| Tubulin α-1C chain | K1QII6_CRAGI | 51 | 744 | 47 | 14 | 2.74 | ||
| 4 | Paramyosin | K1QTC1_CRAGI | 100 | 98 | 6288 | 74 | 69 | 61.54 |
| Alpha-actinin | K1RH58_CRAGI | 102 | 1480 | 56 | 38 | 4.44 | ||
| Filamin | K1PW06_CRAGI | 326 | 1711 | 28 | 50 | 1.00 | ||
| 5 | Filamin | K1PW06_CRAGI | 150 | 326 | 7159 | 61 | 151 | 7.06 |
| Clathrin heavy chain | K1PNR3_CRAGI | 193 | 1660 | 44 | 60 | 2.22 | ||
| Spectrin α chain | K1R401_CRAGI | 287 | 1905 | 45 | 77 | 1.69 | ||
| Heated | ||||||||
| 6 | Tropomyosin | B7XC66_CRAGI | 36 | 33 | 1570 | 59 | 21 | 33.41 |
| Myosin heavy chain | K1RSS3_CRAGI | 230 | 1988 | 27 | 44 | 1.56 | ||
| Filamin | K1PW06_CRAGI | 326 | 635 | 16 | 23 | 0.32 | ||
| 7 | Tropomyosin | B7XC66_CRAGI | 39 | 33 | 3535 | 57 | 21 | 60.09 |
| Troponin T | K1QPC9_CRAGI | 21 | 522 | 74 | 10 | 7.17 | ||
| Non-neuronal cytoplasmic intermediate filament protein | K1PBC0_CRAGI | 70 | 410 | 35 | 15 | 1.19 | ||
| 8 | Tropomyosin | B7XC66_CRAGI | 50 | 33.1 | 822 | 52 | 13 | 5.77 |
| Non-neuronal cytoplasmic intermediate filament protein | K1PBC0_CRAGI | 69.6 | 1599 | 49 | 29 | 5.32 | ||
| Adipophilin | K1PJC1_CRAGI | 54.4 | 791 | 41 | 16 | 2.44 | ||
| 9 | Paramyosin | K1QTC1_CRAGI | 100 | 98 | 7125 | 63 | 46 | 20.17 |
| Filamin | K1PW06_CRAGI | 326 | 5441 | 47 | 107 | 2.60 | ||
| Myosin heavy chain | K1RSS3_CRAGI | 230 | 4644 | 45 | 115 | 1.71 | ||
| 10 | Myosin heavy chain | K1R1B3_CRAGI | 120 | 80 | 3998 | 58 | 36 | 11.60 |
| Paramyosin | K1QTC1_CRAGI | 98.1 | 1523 | 45 | 36 | 2.15 | ||
| Filamin | K1PW06_CRAGI | 326.2 | 1796 | 23 | 53 | 0.73 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nugraha, R.; Ruethers, T.; Johnston, E.B.; Rolland, J.M.; O’Hehir, R.E.; Kamath, S.D.; Lopata, A.L. Effects of Extraction Buffer on the Solubility and Immunoreactivity of the Pacific Oyster Allergens. Foods 2021, 10, 409. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10020409
Nugraha R, Ruethers T, Johnston EB, Rolland JM, O’Hehir RE, Kamath SD, Lopata AL. Effects of Extraction Buffer on the Solubility and Immunoreactivity of the Pacific Oyster Allergens. Foods. 2021; 10(2):409. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10020409
Chicago/Turabian StyleNugraha, Roni, Thimo Ruethers, Elecia B. Johnston, Jennifer M. Rolland, Robyn E. O’Hehir, Sandip D. Kamath, and Andreas L. Lopata. 2021. "Effects of Extraction Buffer on the Solubility and Immunoreactivity of the Pacific Oyster Allergens" Foods 10, no. 2: 409. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10020409