Atherosclerosis Development and Aortic Contractility in Hypercholesterolemic Rabbits Supplemented with Two Different Flaxseed Varieties
Abstract
:1. Introduction
2. Materials and Methods
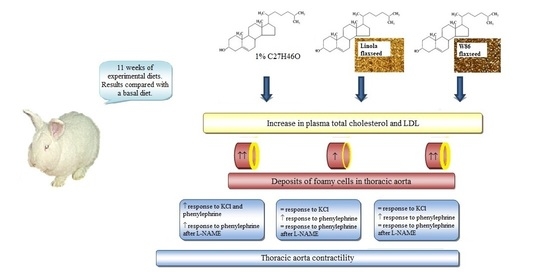
2.1. Animals and Experimental Design
2.2. Plant Material
2.3. Blood Sampling and Analysis
2.4. Tissue Preparation
2.5. Histopathology
2.6. Aorta Contractile Studies
2.7. Data Analysis
3. Results
3.1. Plasma Lipid Profile
3.2. Histopathology
3.3. Aorta Contractility
4. Discussion
4.1. Plasma Lipid Profile
4.2. Histopathology
4.3. Aorta Contractility
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Ethical Statement
References
- European Heart Network. European Cardiovascular Disease Statistics. 2017. Available online: http://www.ehnheart.org/cvd-statistics.html (accessed on 15 February 2018).
- Roth, G.A.; Huffman, M.D.; Moran, A.E.; Feigin, V.; Mensah, G.A.; Naghavi, M.; Murray, C.J. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation 2015, 27, 1667–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barquera, S.; Pedroza-Tobías, A.; Medina, C.; Hernández-Barrera, L.; Bibbins-Domingo, K.; Lozano, R.; Moran, A.E. Global Overview of the Epidemiology of Atherosclerotic Cardiovascular Disease. Arch. Med. Res. 2015, 46, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Hu, F.B. Dietary Fat and Risk of Cardiovascular Disease: Recent Controversies and Advances. Annu. Rev. Nutr. 2017, 37, 423–446. [Google Scholar] [CrossRef]
- De Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schunemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, 3978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visioli, F.; Poli, A. Fatty Acids and Cardiovascular Risk. Evidence, Lack of Evidence, and Diligence. Nutrients 2020, 12, 3782. [Google Scholar] [CrossRef] [PubMed]
- Praagman, J.; Beulens, J.W.J.; Alssema, M.; Zock, P.L.; Wanders, A.J.; Sluijs, I.; Van der Schouw, Y.T. The association between dietary saturated fatty acids and ischemic heart disease depends on the type and source of fatty acid in the European Prospective Investigation into Cancer and Nutrition–Netherlands cohort. Am. J. Clin. Nutr. 2016, 103, 356–365. [Google Scholar] [CrossRef]
- Marangoni, F.; Novo, G.; Perna, G.; Perrone Filardi, P.; Pirelli, S.; Ceroti, M.; Querci, A.; Poli, A. Omega-6 and omega-3 polyunsaturated fatty acid levels are reduced in whole blood of Italian patients with a recent myocardial infarction: The AGE-IM study. Atherosclerosis 2014, 232, 334–338. [Google Scholar] [CrossRef] [Green Version]
- Parikh, M.; Netticadan, T.; Pierce, G.N. Flaxseed: Its bioactive components and their cardiovascular benefits. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, 146–159. [Google Scholar] [CrossRef]
- Sikand, G.; Severson, T. Top 10 dietary strategies for atherosclerotic cardiovascular risk reduction. Am. J. Prev. Cardiol. 2020, 4, 100106. [Google Scholar] [CrossRef]
- Paschos, G.K.; Yiannakouris, N.; Rallidis, L.S.; Davies, I.; Griffin, B.A.; Panagiotakos, D.B.; Skopouli, F.N.; Votteas, V.; Zampelas, A. Apolipoprotein E genotype in dyslipidemic patients and response of blood lipids and inflammatory markers to alpha-linolenic acid. Angiology 2005, 56, 49–60. [Google Scholar] [CrossRef]
- Baker, E.J.; Valenzuela, C.A.; De Souza, C.O.; Yaqoob, P.; Miles, E.A.; Calder, P.C. Comparative anti-inflammatory effects of plant- and marine-derived omega-3 fatty acids explored in an endothelial cell line. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158662. [Google Scholar] [CrossRef]
- Guichardant, M.; Calzada, C.; Bernoud-Hubac, N.; Lagarde, M.; Véricel, E. Omega-3 polyunsaturated fatty acids and oxygenated metabolism in atherothrombosis. Biochim. Biophys. Acta 2015, 1851, 485–495. [Google Scholar] [CrossRef]
- Sherratt, S.C.R.; Juliano, R.A.; Mason, R.P. Eicosapentaenoic acid (EPA) has optimal chain length and degree of unsaturation to inhibit oxidation of small dense LDL and membrane cholesterol domains as compared to related fatty acids in vitro. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183254. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.O.; Price, J.C.; Bueno, A.A. Beyond Fish Oil Supplementation: The Effects of Alternative Plant Sources of Omega-3 Polyunsaturated Fatty Acids upon Lipid Indexes and Cardiometabolic Biomarkers-An Overview. Nutrients 2020, 12, 3159. [Google Scholar] [CrossRef] [PubMed]
- Khandouzi, N.; Zahedmehr, A.; Mohammadzadeh, A.; Sanati, H.R.; Nasrollahzadeh, J. Effect of flaxseed consumption on flow-mediated dilation and inflammatory biomarkers in patients with coronary artery disease: A randomized controlled trial. Eur. J. Clin. Nutr. 2019, 73, 258–265. [Google Scholar] [CrossRef]
- Prasad, K. Flaxseed: A source of hypocholesterolemic and antiatherogenic agents. Drug News Perspect. 2000, 13, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Żuk, M.; Prescha, A.; Stryczewska, M.; Szopa, J. Engineering flax plant to increase their antioxidant capacity and improve oil composition and stability. J. Agric. Food Chem. 2012, 60, 5003–5012. [Google Scholar] [CrossRef]
- Hasiewicz-Derkacz, K.; Kulma, A.; Czuj, T.; Prescha, A.; Żuk, M.; Grajzer, M.; Łukaszewicz, M.; Szopa, J. Natural phenolics greatly increase flax (Linum usitatissimum) oil stability. BMC Biotechnol. 2015, 30, 15–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogueira, M.S.; Kessuane, M.C.; Lobo Ladd, A.A.; Lobo Ladd, F.V.; Cogliati, B.; Castro, I.A. Effect of long-term ingestion of weakly oxidised flaxseed oil on biomarkers of oxidative stress in LDL-receptor knockout mice. Br. J. Nutr. 2016, 116, 258–269. [Google Scholar] [CrossRef] [Green Version]
- Matusiewicz, M.; Kosieradzka, I.; Zuk, M.; Szopa, J. Genetically modified flax expressing NAP-SsGT1 transgene: Examination of anti-inflammatory action. Int. J. Mol. Sci. 2014, 15, 16741–16759. [Google Scholar] [CrossRef] [Green Version]
- Matusiewicz, M.; Kosieradzka, I.; Sobczak-Filipiak, M.; Zuk, M.; Szopa, J. Transgenic flax overexpressing polyphenols as a potential anti-inflammatory dietary agent. J. Funct. Foods 2015, 14, 299–307. [Google Scholar] [CrossRef]
- Matusiewicz, M.; Kosieradzka, I.; Zuk, M.; Szopa, J. Effect of Dose and Administration Period of Seed Cake of Genetically Modified and Non-Modified Flax on Selected Antioxidative Activities in Rats. Int. J. Mol. Sci. 2015, 16, 14259–14275. [Google Scholar] [CrossRef] [Green Version]
- Króliczewska, B.; Miśta, D.; Ziarnik, A.; Żuk, M.; Szopa, J.; Pecka-Kiełb, E.; Zawadzki, W.; Króliczewski, J. The effects of seed from Linum usitatissimum cultivar with increased phenylpropanoid compounds and hydrolysable tannin in a high cholesterol-fed rabbit. Lipids Health Dis. 2018, 17, 76. [Google Scholar] [CrossRef] [Green Version]
- Cavalcante, J.L.; Lima, J.A.; Redheuil, A.; Al-Mallah, M.H. Aortic stiffness: Current understanding and future directions. J. Am. Coll. Cardiol. 2011, 57, 1511–1522. [Google Scholar] [CrossRef] [Green Version]
- Liao, S.; McLachlan, C.S. Cholesterol Efflux: Does It Contribute to Aortic Stiffening? J. Cardiovasc. Dev. Dis. 2018, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Zuk, M.; Działo, M.; Richter, D.; Dymińska, L.; Matuła, J.; Kotecki, A.; Hanuza, J.; Szopa, J. Chalcone Synthase (CHS) Gene Suppression in Flax Leads to Changes in Wall Synthesis and Sensing Genes, Cell Wall Chemistry and Stem Morphology Parameters. Front. Plant Sci. 2016, 7, 894. [Google Scholar] [CrossRef] [Green Version]
- Brant, N.M.F.; Gasparotto, F.M.; Araújo, V.O.; Maraschin, C.J.; Ribeiro, R.C.L.; Lourenço, B.E.L.; Cardozo Junior, E.L.; Gasparotto Junior, A. Cardiovascular protective effects of Casearia sylvestris Swartzin Swiss and C57BL/6LDLr-null mice undergoing high fat diet. J. Ethnopharmacol. 2014, 154, 419–427. [Google Scholar] [CrossRef] [PubMed]
- El-Sheakh, A.R.; Ghoneim, H.A.; Suddek, G.M.; Ammar, S.M. Antioxidant and anti-inflammatory effects of flavocoxid in high-cholesterol-fed rabbits. Naunyn-Schmiedebergs Arch. Pharmacol. 2015, 388, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Hauzer, W.; Bujok, J.; Czerski, A.; Rusiecka, A.; Pecka, E.; Gnus, J.; Zawadzki, W.; Witkiewicz, W. β-adrenergic antagonists influence abdominal aorta contractility by mechanisms not involving β-adrenergic receptors. Folia Biol. 2014, 62, 243–250. [Google Scholar] [CrossRef]
- Lemcke-Norojärvi, M.; Kamal-Eldin, A.; Appelqvist, L.A.; Dimberg, L.H.; Öhrvall, M.; Vessby, B. Corn and Sesame Oils Increase Serum γ-Tocopherol Concentrations in Healthy Swedish Women. J. Nutr. 2001, 131, 1195–1201. [Google Scholar] [CrossRef] [Green Version]
- Prasad, K. Dietary flax seed in prevention of hypercholesterolemic atherosclerosis. Atherosclerosis 1997, 132, 69–76. [Google Scholar] [CrossRef]
- Prasad, K.; Mantha, S.V.; Muir, A.D.; Wescott, N.D. Reduction of hypercholesterolemic atherosclerosis by CDC-flaxseed with very low alpha-linolenic acid. Atherosclerosis 1998, 136, 367–375. [Google Scholar] [CrossRef]
- Prasad, K.; Khan, A.S.; Shoker, M. Flaxseed and Its Components in Treatment of Hyperlipidemia and Cardiovascular Disease. Int. J. Angiol. 2020, 29, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Dupasquier, C.M.C.; Weber, A.M.; Ander, B.P.; Rampersad, P.P.; Steigerwald, S.; Wigle, J.T.; Mitchell, R.W.; Kroeger, E.A.; Gilchrist, J.S.C.; Moghadasian, M.M.; et al. Effects of dietary flaxseed on vascular contractile function and atherosclerosis during prolonged hypercholesterolemia in rabbits. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, 2987–2996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, K. Flaxseed and cardiovascular health. J. Cardiovasc. Pharmacol. 2009, 54, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Francis, A.A.; Deniset, J.F.; Austria, J.A.; LaValleé, R.K.; Maddaford, G.G.; Hedley, T.E.; Dibrov, E.; Pierce, G.N. Effects of dietary flaxseed on atherosclerotic plaque regression. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, 1743–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods- a review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Arora, R.R.; Singh, M.; Khosla, S. Eicosapentaenoic Acid Versus Docosahexaenoic Acid as Options for Vascular Risk Prevention: A Fish Story. Am. J. Ther. 2016, 23, 905–910. [Google Scholar] [CrossRef]
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef]
- Wang, Y.; Oram, J.F. Unsaturated fatty acids inhibit cholesterol efflux from macrophages by increasing degradation of ATP-binding cassette transporter A1. J. Biol. Chem. 2002, 277, 5692–5697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Oram, J.F. Unsaturated fatty acids phosphorylate and destabilize ABCA1 through a protein kinase C delta pathway. J. Lipid Res. 2007, 48, 1062–1068. [Google Scholar] [PubMed] [Green Version]
- Uehara, Y.; Miura, S.; von Eckardstein, A.; Abe, S.; Fujii, A.; Matsuo, Y.; Rust, S.; Lorkowski, S.; Assmann, G.; Yamada, T.; et al. Unsaturated fatty acids suppress the expression of the ATP-binding cassette transporter G1 (ABCG1) and ABCA1 genes via an LXR/RXR responsive element. Atherosclerosis 2007, 191, 11–21. [Google Scholar] [CrossRef]
- Zhang, J.; Grieger, J.A.; Kris-Etherton, P.M.; Thompson, J.T.; Gillies, P.J.; Fleming, J.A.; Vanden Heuvel, J.P. Walnut oil increases cholesterol efflux through inhibition of stearoyl CoA desaturase 1 in THP-1 macrophage-derived foam cells. Nutr. Metab. 2011, 26, 8–61. [Google Scholar] [CrossRef] [Green Version]
- Spartano, N.L.; Lamon-Fava, S.; Matthan, N.R.; Obin, M.S.; Greenberg, A.S.; Lichtenstein, A.H. Linoleic acid suppresses cholesterol efflux and ATP-binding cassette transporters in murine bone marrow-derived macrophages. Lipids 2014, 49, 415–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Song, Z.; Wang, F.; Xia, H.; Liu, H.; Shu, G.; Lu, H.; Wang, S.; Sun, G. Effects of Linoleic and Alpha-Linolenic Ratios and Concentrations on In Vitro Endothelial Cell Responses. Eur. J. Lipid Sci. Technol. 2018, 120, 1700468. [Google Scholar] [CrossRef]
- Zanwar, A.A.; Hegde, M.V.; Rojatkar, S.R.; Sonawane, K.B.; Rajamohanan, P.R.; Bodhankar, S.L. Isolation, characterization and antihyperlipidemic activity of secoisolariciresinol diglucoside in poloxamer-407-induced experimental hyperlipidemia. Pharm. Biol. 2014, 52, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Jadhav, A. Prevention and treatment of atherosclerosis with flaxseed-derived compound secoisolariciresinol diglucoside. Curr. Pharm. Des. 2016, 22, 214–220. [Google Scholar] [CrossRef]
- Ito, F. Polyphenols can Potentially Prevent Atherosclerosis and Cardiovascular Disease by Modulating Macrophage Cholesterol Metabolism. Curr. Mol. Pharmacol. 2021, 14, 175–190. [Google Scholar] [CrossRef]
- Ge, Z.; Zhang, M.; Deng, X.; Zhu, W.; Li, K.; Li, C. Persimmon tannin promoted macrophage reverse cholesterol transport through inhibiting ERK1/2 and activating PPARγ both in vitro and in vivo. J. Funct. Foods 2017, 38, 338–348. [Google Scholar] [CrossRef]
- Ekambaram, S.P.; Babu, K.B.; Perumal, S.S.; Rajendran, D. Repeated oral dose toxicity study on hydrolysable tannin rich fraction isolated from fruit pericarps of Terminalia chebula Retz in Wistar albino rats. Regul. Toxicol. Pharmacol. 2018, 92, 182–188. [Google Scholar] [CrossRef]
- Cavieres, V.; Valdes, K.; Moreno, B.; Moore-Carrasco, R.; Gonzalez, D.R. Vascular hypercontractility and endothelial dysfunction before development of atherosclerosis in moderate dyslipidemia: Role for nitric oxide and interleukin-6. Am. J. Cardiovasc. Dis. 2014, 4, 114–122. [Google Scholar]
- Van Assche, T.; Fransen, P.; Guns, P.J.; Herman, A.G.; Bult, H. Altered Ca2+ handling of smooth muscle cells in aorta of apolipoprotein E-deficient mice before development of atherosclerotic lesions. Cell Calcium. 2007, 41, 295–302. [Google Scholar] [CrossRef]
- Merkel, L.A.; Rivera, L.M.; Bilder, G.E.; Perrone, M.H. Differential alteration of vascular reactivity in rabbit aorta with modest elevation of serum cholesterol. Circ. Res. 1990, 67, 550–555. [Google Scholar] [CrossRef] [Green Version]
- Leal, M.A.S.; Dias, A.T.; Porto, M.L.; Brun, B.F.; Gava, A.L.; Meyrelles, S.S.; Gil-Longo, J.; Campos-Toimil, M.; Pereira, T.M.C.; Vasquez, E.C. Sildenafil (Viagra®) Prevents Cox-1/ TXA2 Pathway-Mediated Vascular Hypercontractility in ApoE−/− Mice. Cell Physiol. Biochem. 2017, 44, 1796–1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, T.; Miyamori, K.; Kobayashi, T.; Kamata, K. Apocynin normalizes hyperreactivity to phenylephrine in mesenteric arteries from cholesterol-fed mice by improving endothelium-derived hyperpolarizing factor response. Free Radic. Biol. Med. 2006, 41, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Palla, A.H.; Khan, N.A.; Bashir, S.; Ur-Rehman, N.; Iqbal, J.; Gilani, A.H. Pharmacological basis for the medicinal use of Linum usitatissimum (Flaxseed) in infectious and non-infectious diarrhea. J. Ethnopharmacol. 2015, 160, 61–68. [Google Scholar] [CrossRef]
- Leal, M.A.; Balarini, C.M.; Dias, A.T.; Porto, M.L.; Gava, A.L.; Pereira, T.M.; Meyrelles, S.S.; Vasquez, E.C. Mechanisms of enhanced vasoconstriction in the mouse model of atherosclerosis: The beneficial effects of sildenafil. Curr. Pharm. Biotechnol. 2015, 16, 517–530. [Google Scholar] [CrossRef]
- Tabernero, A.; Giraldo, J.; Vila, E. Effect of NG-nitro-L-arginine methyl ester (L-NAME) on functional and biochemical alpha 1-adrenoceptor-mediated responses in rat blood vessels. Br. J. Pharmacol. 1996, 117, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Sherif, I.O. Secoisolariciresinol diglucoside in high-fat diet and streptozotocin-induced diabetic nephropathy in rats: A possible renoprotective effect. J. Physiol. Biochem. 2014, 70, 961–969. [Google Scholar] [CrossRef]
- Nunes, D.O.; Almenara, C.C.P.; Broseghini-Filho, G.B. Flaxseed oil increases aortic reactivity to phenylephrine through reactive oxygen species and the cyclooxygenase-2 pathway in rats. Lipids Health Dis. 2014, 13, 107. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhang, M.; Mukosera, G.T.; Borchardt, D.; Li, Q.; Tipple, T.E.; Ahmed, A.S.I.; Power, G.G.; Blood, A.B. L-NAME releases nitric oxide and potentiates subsequent nitroglycerin-mediated vasodilation. Redox Biol. 2019, 26, 101–238. [Google Scholar] [CrossRef] [PubMed]
- Davda, R.K.; Stepniakowski, K.T.; Lu, G.; Ullian, M.E.; Goodfriend, T.L.; Egan, B.M. Oleic Acid Inhibits Endothelial Nitric Oxide Synthase by a Protein Kinase C–Independent Mechanism. Hypertension 1995, 26, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Garban, J.; Jones, P.J.; Vanden Heuvel, J.; Lamarche, B.; Jenkins, D.J.; Connelly, P.W.; Couture, P.; Pu, S.; Fleming, J.A.; et al. Diets Low in Saturated Fat with Different Unsaturated Fatty Acid Profiles Similarly Increase Serum-Mediated Cholesterol Efflux from THP-1 Macrophages in a Population with or at Risk for Metabolic Syndrome: The Canola Oil Multicenter Intervention Trial. J. Nutr. 2018, 148, 721–728. [Google Scholar] [CrossRef] [PubMed]




| Item (μg/gFW) | Linola | W86 |
|---|---|---|
| 16:0 | 11.33 ± 0.5 | 13.60 ± 0.5 |
| 16:1 | 0.16 ± 0.02 | 0.22 ± 0.03 |
| 16:2 | 0.12 ± 0.02 | 0.17 ± 0.02 |
| 16:3 (n-3 FA) | 0.10 ± 0.01 | 0.10 ± 0.01 |
| 18:0 | 6.38 ± 0.25 | 7.63 ± 0.25 |
| 18:1(OA, n-9 FA) | 32.57 ± 0.75 | 40.05 ± 0.68 |
| 18:2 (LA, n-6 FA) | 158.57 ± 3.85 | 153.91 ± 4.55 |
| 18:3 (ALA, n-3 FA) | 3.64 ± 0.5 | 105.70 ± 2.56 |
| 20:0 | 0.26 ± 0.01 | 0.26 ± 0.01 |
| 20:1(n-9 FA) | 0.19 ± 0.01 | 0.19 ± 0.01 |
| 22:0 | 0.12 ± 0.01 | 0.14 ± 0.01 |
| 22:1(n-9 FA) | 0.10 ± 0.01 | 0.18 ± 0.02 |
| SFA | 18.13 ± 0.97 | 21.70 ± 1.05 |
| MUFA | 33.02 ± 0.76 | 40.64 ± 0.73 |
| PUFA | 162.21 ± 2.86 | 259.61 ± 3.45 |
| n-6/n-3 | 43/1 | 1.5/1 |
| n-9/n-6/n-3 | 9/43/1 | 1/4/2.5 |
| Total | 213.57 ± 3.91 | 322.22 ± 3.25 |
| Item (mg/gFW) | Linola | W86 |
|---|---|---|
| Ferulic acid and glucoside | 2.131 ± 0.048 | 2.382 ± 0.043 |
| Coumaric acid and glucoside | 1.400 ± 0.059 | 1.626 ± 0.024 |
| Caffeic acid and glucoside | 0.782 ± 0.019 | 1.060 ± 0.092 |
| Phenolic acids (total) | 4.313 ± 0.126 | 5.068 ± 0.159 |
| Vitexin | 0.028 ± 0.05 | 0.016 ± 0.001 |
| Secoisolariciresinol diglucoside (SDG) | 13.31 ± 0.387 | 14.36 ± 0.020 |
| Coniferyl aldehyde | 0.002 ± 0.001 | 0.003 ± 0.001 |
| Proanthocyanidin | 0.025 ± 0.002 | 0.052 ± 0.008 |
| Hydrolysable tannins | 0.048 ± 0.001 | 0.279 ± 0.013 |
| Parameter | C | CH | W | L | |
|---|---|---|---|---|---|
| n | 5 | 5 | 4 | 5 | |
| TChol, mmol/L | 0.65 ± 0.12 a | 13.05 ± 2.33 b | 12.17 ± 1.96 b | 12.07 ± 2.85 b | |
| HDL, mmol/L | 0.47 ± 0.11 | 0.74 ± 0.08 | 0.58 ± 0.05 | 0.61 ± 0.11 | |
| LDL, mmol/L | 0.18 ± 0.02 a | 7.10 ± 1.40 b | 6.04 ± 1.11 b | 6.42 ± 1.73 b | |
| TG, mmol/L | 0.53 ± 0.09 | 0.91 ± 0.12 | 0.77 ± 0.10 | 1.03 ± 0.29 |
| Group | n | Foamy Cell Deposit Thickness [µm] |
|---|---|---|
| C | 5 | 1.13 ± 1.13 a |
| CH | 5 | 22.43 ± 5.16 b |
| W | 4 | 27.79 ± 6.43 b |
| L | 5 | 11.75 ± 3.38 a,b |
| C | CH | W | L | |||
|---|---|---|---|---|---|---|
| a | KCl 60 mM | n | 23 | 22 | 19 | 20 |
| Emax [g] | 0.60 ± 0.07 a | 1.01 ± 0.12 b | 0.75 ± 0.06 a,b | 0.70 ± 0.06 a | ||
| b | PHE | n | 6 | 5 | 5 | 5 |
| Emax [g] | 0.97 ± 0.17 a | 1.73 ± 0.46 b | 1.46 ± 0.18 a,b | 1.54 ± 0.16 a,b | ||
| pK | −5.77 ± 0.18 | −6.07 ± 0.17 | −5.91 ± 0.24 | −6.18 ± 0.07 | ||
| PHE + L-NAME 10 µM | n | 6 | 5 | 4 | 5 | |
| Emax [g] | 1.11 ± 0.11 b | 2.02 ± 0.23 a,⌠ | 1.41 ± 0.25 a,b | 1.28 ± 0.17 a,b,⌠ | ||
| pK | −5.94 ± 0.11 | −6.19 ± 0.16 | −6.18 ± 0.16 | −6.06 ± 0.06 | ||
| PHE + L-NAME 100 µM | n | 6 | 6 | 4 | 5 | |
| Emax [g] | 1.32 ± 0.23 | 2.13 ± 0.43 | 1.39 ± 0.32 | 1.49 ± 0.12 | ||
| pK | −6.03 ± 0.10 | −6.23 ± 0.15 | −6.02 ± 0.19 | −6.20 ± 0.10 | ||
| c | SNP | n | 20 | 21 | 15 | 19 |
| Emax [%] | −18.1 ± 4.6 | −15.3 ± 8.3 | −13.2 ± 6.0 | −8.3 ± 3.8 | ||
| pK | 5.65 ± 0.24 | 4.97 ± 0.69 | 5.68 ± 0.06 | 5.22 ± 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bujok, J.; Miśta, D.; Wincewicz, E.; Króliczewska, B.; Dzimira, S.; Żuk, M. Atherosclerosis Development and Aortic Contractility in Hypercholesterolemic Rabbits Supplemented with Two Different Flaxseed Varieties. Foods 2021, 10, 534. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10030534
Bujok J, Miśta D, Wincewicz E, Króliczewska B, Dzimira S, Żuk M. Atherosclerosis Development and Aortic Contractility in Hypercholesterolemic Rabbits Supplemented with Two Different Flaxseed Varieties. Foods. 2021; 10(3):534. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10030534
Chicago/Turabian StyleBujok, Jolanta, Dorota Miśta, Edyta Wincewicz, Bożena Króliczewska, Stanisław Dzimira, and Magdalena Żuk. 2021. "Atherosclerosis Development and Aortic Contractility in Hypercholesterolemic Rabbits Supplemented with Two Different Flaxseed Varieties" Foods 10, no. 3: 534. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10030534