Characterization of Collagen Structure in Normal, Wooden Breast and Spaghetti Meat Chicken Fillets by FTIR Microspectroscopy and Histology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chicken Fillets
2.2. Thin Section Sample Preparation
2.3. Histology
2.4. FTIR Micro Spectroscopy
2.5. Polarized FTIR Imaging
2.6. Spectral Data Analysis
3. Results
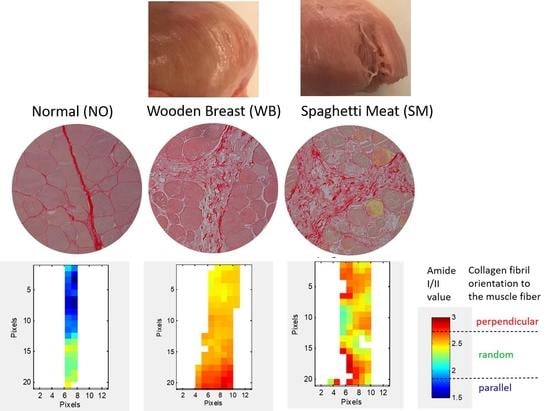
3.1. Histological Characterization of Skeletal Muscle Abnormalities
3.2. FTIR Microspectroscopy
3.3. Polarized FTIR Imaging
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Approval and Consent to Participate
References
- Dransfield, E.; Sosnicki, A. Relationship between muscle growth and poultry meat quality. Poult. Sci. 1999, 78, 743–746. [Google Scholar] [CrossRef]
- Sihvo, H.K.; Immonen, K.; Puolanne, E. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet. Pathol. 2014, 51, 619–623. [Google Scholar] [CrossRef]
- Baldi, G.; Soglia, F.; Mazzoni, M.; Sirri, F.; Canonico, L.; Babini, E.; Laghi, L.; Cavani, C.; Petracci, M. Implications of white striping and spaghetti meat abnormalities on meat quality and histological features in broilers. Animal 2018, 12, 164–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velleman, S.G.; Clark, D.L. Histopathologic and Myogenic Gene Expression Changes Associated with Wooden Breast in Broiler Breast Muscles. Avian Dis. 2015, 59, 410–418. [Google Scholar] [CrossRef]
- Serrano, A.L.; Muñoz-Cánoves, P. Regulation and dysregulation of fibrosis in skeletal muscle. Exp. Cell Res. 2010, 316, 3050–3058. [Google Scholar] [CrossRef]
- Cavani, C.; Babini, E.; Sirri, F.; Soglia, F.; Di Nunzio, M.; Petracci, M.; Mudalal, S.; Mazzoni, M. Histology, composition, and quality traits of chicken Pectoralis major muscle affected by wooden breast abnormality. Poult. Sci. 2015, 95, 651–659. [Google Scholar] [CrossRef]
- Wold, J.P.; Veiseth-Kent, E.; Høst, V.; Løvland, A. Rapid on-line detection and grading of wooden breast myopathy in chicken fillets by near-infrared spectroscopy. PLoS ONE 2017, 12, e0173384. [Google Scholar] [CrossRef]
- Wold, J.P.; Måge, I.; Løvland, A.; Sanden, K.W.; Ofstad, R. Near-infrared spectroscopy detects woody breast syndrome in chicken fillets by the markers protein content and degree of water binding. Poult. Sci. 2019, 98, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Soglia, F.; Gao, J.; Mazzoni, M.; Puolanne, E.; Cavani, C.; Petracci, M.; Ertbjerg, P. Superficial and deep changes of histology, texture and particle size distribution in broiler wooden breast muscle during refrigerated storage. Poult. Sci. 2017, 96, 3465–3472. [Google Scholar] [CrossRef]
- Velleman, S.G. Recent Developments in Breast Muscle Myopathies Associated with Growth in Poultry. Annu. Rev. Anim. Biosci. 2019, 7, 289–308. [Google Scholar] [CrossRef]
- Clark, D.L.; Velleman, S.G. Spatial influence on breast muscle morphological structure, myofiber size, and gene expression associated with the wooden breast myopathy in broilers. Poult. Sci. 2016, 95, 2930–2945. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [Green Version]
- Gillies, A.R.; Chapman, M.A.; Bushong, E.A.; Deerinck, T.J.; Ellisman, M.H.; Lieber, R.L. High resolution three-dimensional reconstruction of fibrotic skeletal muscle extracellular matrix. J. Physiol. 2017, 595, 1159–1171. [Google Scholar] [CrossRef]
- Chapman, M.A.; Pichika, R.; Lieber, R.L. Collagen crosslinking does not dictate stiffness in a transgenic mouse model of skeletal muscle fibrosis. J. Biomech. 2015, 48, 375–378. [Google Scholar] [CrossRef] [Green Version]
- Velleman, S.G.; Clark, D.L.; Tonniges, J.R. Fibrillar Collagen Organization Associated with Broiler Wooden Breast Fibrotic Myopathy. Avian Dis. 2017, 61, 481–490. [Google Scholar] [CrossRef]
- Tonniges, J.R.; Clark, D.L.; Velleman, S.G. The Effect of the Wooden Breast Fibrotic Myopathy in Broilers on Fibrillar Collagen Organization and Decorin-Collagen Binding. Avian Dis. 2019, 63, 48–60. [Google Scholar] [CrossRef]
- Thygesen, L.G.; Løkke, M.M.; Micklander, E.; Engelsen, S.B. Vibrational microspectroscopy of food. Raman vs. FT-IR. Trends Food Sci. Technol. 2003, 14, 50–57. [Google Scholar] [CrossRef]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böcker, U.; Ofstad, R.; Bertram, H.C.; Egelandsdal, B.; Kohler, A. Salt-Induced Changes in Pork Myofibrillar Tissue Investigated by FT-IR Microspectroscopy and Light Microscopy. J. Agric. Food Chem. 2006, 54, 6733–6740. [Google Scholar] [CrossRef] [PubMed]
- Sanden, K.W.; Kohler, A.; Afseth, N.K.; Bocker, U.; Ronning, S.B.; Liland, K.H.; Pedersen, M.E. The use of Fourier-transform infrared spectroscopy to characterize connective tissue components in skeletal muscle of Atlantic cod (Gadus morhua L.). J. Biophotonics. 2019, 12, e201800436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, A.J.; Paul, R.G.; Knott, L. Mechanisms of maturation and ageing of collagen. Mech. Ageing Dev. 1998, 106, 1–56. [Google Scholar] [CrossRef]
- Paschalis, E.P.; Verdelis, K.; Doty, S.B.; Boskey, A.L.; Mendelsohn, R.; Yamauchi, M. Spectroscopic Characterization of Collagen Cross-Links in Bone. J. Bone Min. Res. 2001, 16, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, E.P.; Gamsjaeger, S.; Tatakis, D.N.; Hassler, N.; Robins, S.P.; Klaushofer, K. Fourier transform Infrared spectroscopic characterization of mineralizing type I collagen enzymatic trivalent cross-links. Calcif. Tissue Int. 2015, 96, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Li, G.; Doty, S.B.; Camacho, N.P. A novel method for determination of collagen orientation in cartilage by Fourier transform infrared imaging spectroscopy (FT-IRIS). Osteoarthr. Cartil. 2005, 13, 1050–1058. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.; Eklouh-Molinier, C.; Sebiskveradze, D.; Feru, J.; Terryn, C.; Manfait, M.; Brassart-Pasco, S.; Piot, O. Changes of skin collagen orientation associated with chronological aging as probed by polarized-FTIR micro-imaging. Analyst 2014, 139, 2482–2488. [Google Scholar] [CrossRef]
- Junqueira, L.C.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef]
- Ganganna, K.; Shetty, P.; Shroff, S.E. Collagen in histologic stages of oral submucous fibrosis: A polarizing microscopic study. J. Oral Maxillofac. Pathol. JOMFP 2012, 16, 162–166. [Google Scholar] [CrossRef] [Green Version]
- Tfayli, A.; Piot, O.; Durlach, A.; Bernard, P.; Manfait, M. Discriminating nevus and melanoma on paraffin-embedded skin biopsies using FTIR microspectroscopy. Biochim. Biophys. Acta (BBA) Gen. Subj. 2005, 1724, 262–269. [Google Scholar] [CrossRef]
- Afseth, N.K.; Kohler, A. Extended multiplicative signal correction in vibrational spectroscopy, a tutorial. Chemom. Intell. Lab. Syst. 2012, 117, 92–99. [Google Scholar] [CrossRef]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Farlay, D.; Duclos, M.-E.; Gineyts, E.; Bertholon, C.; Viguet-Carrin, S.; Nallala, J.; Sockalingum, G.D.; Bertrand, D.; Roger, T.; Hartmann, D.J.; et al. The Ratio 1660/1690 cm−1 Measured by Infrared Microspectroscopy Is Not Specific of Enzymatic Collagen Cross-Links in Bone Tissue. PLoS ONE 2011, 6, e28736. [Google Scholar] [CrossRef] [Green Version]
- Bychkov, S.M.; Kuz’mina, S.A. Tissue proteoglycans studied by infrared spectroscopy. Bull. Exp. Biol. Med. 1992, 114, 1245–1249. [Google Scholar] [CrossRef]
- Bychkov, S.M.; Kuz’mina, S.A. Comparative study of infrared spectra of proteoglycans. Bull. Exp. Biol. Med. 1991, 112, 1571–1573. [Google Scholar] [CrossRef]
- Payne, K.J.; Veis, A. Fourier transform ir spectroscopy of collagen and gelatin solutions: Deconvolution of the amide I band for conformational studies. Biopolymers 1988, 27, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.; Mantsch, H.H. The Use and Misuse of FTIR Spectroscopy in the Determination of Protein Structure. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Servaty, R.; Schiller, J.; Binder, H.; Arnold, K. Hydration of polymeric components of cartilage—An infrared spectroscopic study on hyaluronic acid and chondroitin sulfate. Int. J. Biol. Macromol. 2001, 28, 121–127. [Google Scholar] [CrossRef]
- Barth, A.; Zscherp, C. What vibrations tell about proteins. Q. Rev. Biophys. 2002, 35, 369–430. [Google Scholar] [CrossRef] [PubMed]
- Velleman, S.G. Relationship of Skeletal Muscle Development and Growth to Breast Muscle Myopathies: A Review. Avian Dis. 2015, 59, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Baldi, G.; Soglia, F.; Laghi, L.; Tappi, S.; Rocculi, P.; Tavaniello, S.; Prioriello, D.; Mucci, R.; Maiorano, G.; Petracci, M. Comparison of quality traits among breast meat affected by current muscle abnormalities. Food Res. Int. 2019, 115, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Canuto, H.C.; Fishbein, K.W.; Huang, A.; Doty, S.B.; Herbert, R.A.; Peckham, J.; Pleshko, N.; Spencer, R.G. Characterization of skin abnormalities in a mouse model of osteogenesis imperfecta using high resolution magnetic resonance imaging and Fourier transform infrared imaging spectroscopy. NMR Biomed. 2012, 25, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Perez-Puyana, V.; Ostos, F.J.; López-Cornejo, P.; Romero, A.; Guerrero, A. Assessment of the denaturation of collagen protein concentrates using different techniques. Biol. Chem. 2019, 400, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Petracci, M.; Soglia, F.; Madruga, M.; Carvalho, L.; Ida, E.; Estevez, M. Wooden-Breast, White Striping, and Spaghetti Meat: Causes, Consequences and Consumer Perception of Emerging Broiler Meat Abnormalities. Compr. Rev. Food Sci. Food Saf. 2019, 18, 565–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottje, W.G.; Lassiter, K.R.; Kuttappan, V.A.; Hudson, N.J.; Owens, C.M.; Abasht, B.; Dridi, S.; Kong, B.C. Upstream Regulator Analysis of Wooden Breast Myopathy Proteomics in Commercial Broilers and Comparison to Feed Efficiency Proteomics in Pedigree Male Broilers. Foods 2021, 10, 104. [Google Scholar] [CrossRef]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanden, K.W.; Böcker, U.; Ofstad, R.; Pedersen, M.E.; Høst, V.; Afseth, N.K.; Rønning, S.B.; Pleshko, N. Characterization of Collagen Structure in Normal, Wooden Breast and Spaghetti Meat Chicken Fillets by FTIR Microspectroscopy and Histology. Foods 2021, 10, 548. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10030548
Sanden KW, Böcker U, Ofstad R, Pedersen ME, Høst V, Afseth NK, Rønning SB, Pleshko N. Characterization of Collagen Structure in Normal, Wooden Breast and Spaghetti Meat Chicken Fillets by FTIR Microspectroscopy and Histology. Foods. 2021; 10(3):548. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10030548
Chicago/Turabian StyleSanden, Karen Wahlstrøm, Ulrike Böcker, Ragni Ofstad, Mona Elisabeth Pedersen, Vibeke Høst, Nils Kristian Afseth, Sissel Beate Rønning, and Nancy Pleshko. 2021. "Characterization of Collagen Structure in Normal, Wooden Breast and Spaghetti Meat Chicken Fillets by FTIR Microspectroscopy and Histology" Foods 10, no. 3: 548. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10030548