Highly-Efficient Release of Ferulic Acid from Agro-Industrial By-Products via Enzymatic Hydrolysis with Cellulose-Degrading Enzymes: Part I–The Superiority of Hydrolytic Enzymes Versus Conventional Hydrolysis
Abstract
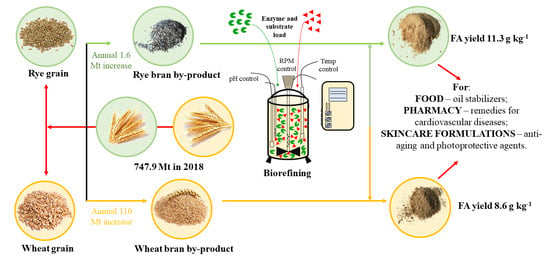
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Plant Material Preparation for Alkaline and Enzymatic Hydrolysis and Analysis of Hydroxycinnamates
2.3. Chemicals and Reagents
2.4. Enzymes
2.5. Hydrolysis of Wheat and Rye Bran
2.5.1. Alkaline-Assisted Hydrolysis
2.5.2. Enzyme-Assisted Hydrolysis
2.6. Generation of Standard Curves
2.7. The HPLC-DAD-ESI/MSn Conditions
2.8. The UPLC-ESI-QTOF/MS Conditions
2.9. Scanning Electron Microscopy (SEM)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Release of FA Using Conventional Alkaline-Assisted Hydrolysis
3.2. Release of FA Using Enzyme-Assisted Hydrolysis with Commercial Cellulolytic Enzymes
3.3. Release of Glucose Using Enzyme-Assisted Hydrolysis with Commercial Cellulolytic Enzymes
3.4. Structural Changes in Wheat and Rye Bran Morphology Induced by Cellulolytic Enzymes
3.5. Release of FA Using Enzyme-Assisted Hydrolysis Accomplished by Glycolytic and Cellulolytic Enzymes
3.6. Release of FA Using Enzyme-Assisted Hydrolysis with Cellulolytic Enzymes in Combination with Feruloyl Esterase
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Radenkovs, V.; Juhnevica-Radenkova, K.; Górnaś, P.; Seglina, D. Non-waste technology through the enzymatic hydrolysis of agro-industrial by-products. Trends Food Sci. Technol. 2018, 77, 64–76. [Google Scholar] [CrossRef]
- FAOSTAT. FAO Stat Database. Global Crops Production Quantity. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 24 June 2020).
- Rahman, A.; Ulven, C.A.; Johnson, M.A.; Durant, C.; Hossain, K.G. Pretreatment of wheat bran for suitable reinforcement in biocomposites. J. Renew. Mater. 2017, 5, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, D.R.; Kammerer, J.; Valet, R.; Carle, R. Recovery of polyphenols from the by-products of plant food processing and application as valuable food ingredients. Food Res. Int. 2014, 65, 2–12. [Google Scholar] [CrossRef]
- Jefremova, O.; Radenkovs, V.; Kunkulberga, D.; Klava, D. Technological properties of dough from wheat flour and fermented bran. Cheminė Technol. 2015, 1, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.L.; Zhang, M.; Zhang, D.H.; Chen, X.L.; Sun, C.Y.; Zhou, B.C.; Zhang, Y.Z. Purification and enzymatic characterization of two β-endoxylanases from Trichoderma sp. K9301 and their actions in xylooligosaccharide production. Bioresour. Technol. 2009, 100, 5230–5236. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Bai, Y.; Huang, H.; Luo, H.; Chen, S.; Fan, Y.; Cai, L.; Yao, B. Utility of thermostable xylanases of mycothermus thermophilus in generating prebiotic xylooligosaccharides. J. Agric. Food Chem. 2017, 65, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, H.; Xue, Y.; Luo, G.; Gan, L.; Liu, J.; Mao, L.; Long, M. High efficiency co-production of ferulic acid and xylooligosaccharides from wheat bran by recombinant xylanase and feruloyl esterase. Biochem. Eng. J. 2017, 120, 41–48. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Balasubashini, M.S.; Rukkumani, R.; Viswanathan, P.; Menon, V.P. Ferulic acid alleviates lipid peroxidation in diabetic rats. Phyther. Res. 2004, 18, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Global Ferulic Acid Market. 2020. Available online: https://www.industryresearch.biz/global-ferulic-acid-market-16039706 (accessed on 2 October 2020).
- Ahuja, K.; Mamtani, K. Global Market Insights. 2019. Available online: https://www.gminsights.com/industry-analysis/natural-ferulic-acid-market (accessed on 4 October 2020).
- Ou, S.; Kwok, K.C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Magnani, C.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic acid: A review of its potential use in medications and cosmetics. Anal. Methods 2014, 6, 3203–3210. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Benoit, I.; Navarro, D.; Marnet, N.; Rakotomanomana, N.; Lesage-Meessen, L.; Sigoillot, J.C.; Asther, M.; Asther, M. Feruloyl esterases as a tool for the release of phenolic compounds from agro-industrial by-products. Carbohydr. Res. 2006, 341, 1820–1827. [Google Scholar] [CrossRef]
- Anson, N.M.; Selinheimo, E.; Havenaar, R.; Aura, A.M.; Mattila, I.; Lehtinen, P.; Bast, A.; Poutanen, K.; Haenen, G.R.M.M. Bioprocessing of wheat bran improves In Vitro bioaccessibility and colonic metabolism of phenolic compounds. J. Agric. Food Chem. 2009, 57, 6148–6155. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef]
- Aarabi, A.; Mizani, M.; Honarvar, M.; Faghihian, H.; Gerami, A. Extraction of ferulic acid from sugar beet pulp by alkaline hydrolysis and organic solvent methods. J. Food Meas. Charact. 2016, 10, 42–47. [Google Scholar] [CrossRef]
- Carvalho, D.O.; Curto, A.F.; Guido, L.F. Determination of phenolic content in different barley varieties and corresponding malts by liquid chromatography-diode array detection-electrospray ionization tandem mass spectrometry. Antioxidants 2015, 4, 563–576. [Google Scholar] [CrossRef] [Green Version]
- Ideia, P.; Sousa-Ferreira, I.; Castilho, P.C. A Novel and simpler alkaline hydrolysis brewer’s spent grain and its (partial) purification. Foods 2020, 9, 600. [Google Scholar] [CrossRef]
- Gomes, H.I.; Mayes, W.M.; Rogerson, M.; Stewart, D.I.; Burked, I.T. Alkaline residues and the environment: A review of impacts, management practices and opportunities. J. Clean. Prod. 2016, 112, 3571–3582. [Google Scholar] [CrossRef] [Green Version]
- Faulds, C.B.; Williaon, G. Release of ferulic acid from wheat bran by a ferulic acid esterase (FAE-III) from Aspergillus niger. Appl. Microbiol. Biotechnol. 1995, 43, 1082–1087. [Google Scholar] [CrossRef]
- Shin, H.-D.; McClendon, S.; Le, T.; Taylor, F.; Chen, R.R. A Complete enzymatic recovery of ferulic acid from corn residues with extracellular enzymes from Neosartorya spinosa NRRL185. Biotechnol. Bioeng. 2006, 95, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Radenkovs, V.; Kviesis, J.; Juhnevica-Radenkova, K.; Valdovska, A.; Püssa, T.; Klavins, M.; Drudze, I. Valorization of wild apple (Malus spp.) by-products as a source of essential fatty acids, tocopherols and phytosterols with antimicrobial activity. Plants 2018, 7, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, R.P. Gravimetric Measurements of Water. In Handbook of Food Analytical Chemistry, Water, Proteins, Enzymes, Lipids, and Carbohydrates; Wrolstad, R.E., Acree, T.E., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Smith, D., Sporns, P., Eds.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2005; pp. 5–33. ISBN 9780471709084. [Google Scholar]
- Zhang, J.; Ding, Y.; Dong, H.; Hou, H.; Zhang, X. Distribution of phenolic acids and antioxidant activities of different bran fractions from three pigmented wheat varieties. J. Chem. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Radenkovs, V.; Klava, D.; Juhnevica-Radenkova, K. Effect of enzymatic hydrolysis on bran microflora. In Proceedings of the Annual 20th International Scientific Conference Proceedings “Research for Rural Development”, Jelgava, Latvia, 21–23 May 2014; Volume 1, pp. 148–154. [Google Scholar]
- Radenkovs, V.; Klava, D.; Krasnova, I. Application of enzymatic treatment to improve the concentration of bioactive compounds and antioxidant potential of wheat and rye bran. In Proceedings of the 9th Baltic Conference on Food Science and Technology “Food for Consumer Well-Being” Foodbalt, Jelgava, Latvia, 8–9 May 2014; pp. 127–132. [Google Scholar]
- Heinzerling, P.; Schrader, F.; Schanze, S. Measurement of enzyme kinetics by use of a blood glucometer: Hydrolysis of sucrose and lactose. J. Chem. Educ. 2012, 89, 1582–1586. [Google Scholar] [CrossRef]
- Ferreres, F.; Grosso, C.; Gil-Izquierdo, A.; Fernandes, A.; Valentão, P.; Andrade, P.B. Comparing the phenolic profile of Pilocarpus pennatifolius Lem. by HPLC-DAD-ESI/MSn with respect to authentication and enzyme inhibition potential. Ind. Crops Prod. 2015, 77, 391–401. [Google Scholar] [CrossRef]
- Salgado, J.M.; Max, B.; Rodríguez-Solana, R.; Domínguez, J.M. Purification of ferulic acid solubilized from agroindustrial wastes and further conversion into 4-vinyl guaiacol by Streptomyces setonii using solid state fermentation. Ind. Crops Prod. 2012, 39, 52–61. [Google Scholar] [CrossRef]
- Ohra-Aho, T.; Niemi, P.; Aura, A.M.; Orlandi, M.; Poutanen, K.; Buchert, J.; Tamminen, T. Structure of brewer’s spent grain lignin and its interactions with gut microbiota In Vitro. J. Agric. Food Chem. 2016, 64, 812–820. [Google Scholar] [CrossRef]
- Ferri, M.; Happel, A.; Zanaroli, G.; Bertolini, M.; Chiesa, S.; Commisso, M.; Guzzo, F.; Tassoni, A. Advances in combined enzymatic extraction of ferulic acid from wheat bran. New Biotechnol. 2020, 56, 38–45. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Marzouki, M.; M’Rabet, Y.; Mezni, M.; Ait Ouazzou, A.; Hosni, K. Enzyme pretreatment improves the recovery of bioactive phytochemicals from sweet basil (Ocimum basilicum L.) leaves and their hydrodistilled residue by-products, and potentiates their biological activities. Arab. J. Chem. 2020, 13, 6451–6460. [Google Scholar] [CrossRef]
- Petit-Benvegnen, M.D.; Saulnier, L.; Rouau, X. Solubilization of arabinoxylans from isolated water-unextractable pentosans and wheat flour doughs by cell-wall-degrading enzymes. Cereal Chem. 1998, 75, 551–556. [Google Scholar] [CrossRef]
- Arrigoni, E. Enzymatic modification of dietary fiber sources. In Handbook of Dietary Fiber; Cho, S.S., Dreher, M.L., Eds.; Taylor and Francis: Oxfordshire, UK, 2001; pp. 268–280. [Google Scholar]
- Yu, J.; Vasanthan, T.; Temelli, F. Analysis of phenolic acids in barley by high-performance liquid chromatography. J. Agric. Food Chem. 2001, 49, 4352–4358. [Google Scholar] [CrossRef]
- Sun, J.; Xu, F.; Lu, J. Barley α-amylase/subtilisin inhibitor shows inhibitory activity against endogenous xylanase isozyme I of malted barley: A novel protein function. J. Food Biochem. 2020, 44, 1–8. [Google Scholar] [CrossRef]
- Das, S.; Wong, A.B.H. Stabilization of ferulic acid in topical gel formulation via nanoencapsulation and pH optimization. Sci. Rep. 2020, 10, 1–18. [Google Scholar] [CrossRef]






| Major Nutrients Profile, g 100 g−1 DW. | |||||||
|---|---|---|---|---|---|---|---|
| Type of Material | Moisture, % | Starch | Crude Lipids | Crude Proteins | Crude Cellulose | Crude HEM | ADL |
| Wheat bran | 4.1 ± 0.1 a | 8.7 ± 0.3 b | 3.1 ± 0.0 b | 17.1 ± 0.8 a | 39.8 ± 4.1 a | 12.9 ± 0.9 a | 9.3 ± 0.0 a |
| Rye bran | 4.1 ± 0.1 a | 18.6 ± 0.1 a | 2.5 ± 0.0 a | 17.0 ± 0.7 a | 33.4 ± 2.1 b | 5.3 ± 0.5 b | 3.3 ± 0.0 b |
| Commercial Enzyme | Declared Activity | Enzyme Activity | Source | EC Number |
|---|---|---|---|---|
| Amylase® AG XXL | 460 AGU g−1 | Glucan-1,4-α-glucosidase | Aspergillus niger | 3.2.1.3 |
| Celluclast® 1.5 L | 700 EGU g−1 | 1,4-β-D-endoglucanase | Trichoderma reesei | 3.2.1.4 |
| Viscozyme® L | 100 FBG g−1 | Endo-1,4-β-xylanase α-L-arabinofuranosidase 1,4-β-D-endoglucanase | Aspergillus aculeatus | 3.2.1.8 3.2.1.55 3.2.1.4 |
| Megazyme™ Feruloyl esterase | 30 U mg−1 | Feruloyl esterase | Rumen microorganism, n.s. | 3.1.1.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juhnevica-Radenkova, K.; Kviesis, J.; Moreno, D.A.; Seglina, D.; Vallejo, F.; Valdovska, A.; Radenkovs, V. Highly-Efficient Release of Ferulic Acid from Agro-Industrial By-Products via Enzymatic Hydrolysis with Cellulose-Degrading Enzymes: Part I–The Superiority of Hydrolytic Enzymes Versus Conventional Hydrolysis. Foods 2021, 10, 782. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10040782
Juhnevica-Radenkova K, Kviesis J, Moreno DA, Seglina D, Vallejo F, Valdovska A, Radenkovs V. Highly-Efficient Release of Ferulic Acid from Agro-Industrial By-Products via Enzymatic Hydrolysis with Cellulose-Degrading Enzymes: Part I–The Superiority of Hydrolytic Enzymes Versus Conventional Hydrolysis. Foods. 2021; 10(4):782. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10040782
Chicago/Turabian StyleJuhnevica-Radenkova, Karina, Jorens Kviesis, Diego A. Moreno, Dalija Seglina, Fernando Vallejo, Anda Valdovska, and Vitalijs Radenkovs. 2021. "Highly-Efficient Release of Ferulic Acid from Agro-Industrial By-Products via Enzymatic Hydrolysis with Cellulose-Degrading Enzymes: Part I–The Superiority of Hydrolytic Enzymes Versus Conventional Hydrolysis" Foods 10, no. 4: 782. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10040782