Application of Broccoli Leaf Powder in Gluten-Free Bread: An Innovative Approach to Improve Its Bioactive Potential and Technological Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Broccoli Leaf Powder
2.2. Preparation of Experimental Gluten-Free Bread
2.3. Characteristics of Experimental Gluten-Free Breads
2.3.1. Determination of Proximal Chemical Composition and Energy Value
2.3.2. Determination of Physical Parameters
- a—the initial weight of batter before baking (g), and
- b—the weight of baked and cooled GFs (g).
2.3.3. Evaluation of Textural Properties
2.4. Evaluation of the Antioxidant Capacity of BLP and GFs
2.4.1. Determination of Total Phenolic Content
2.4.2. Trolox Equivalent Antioxidant Capacity by ABTS Assay
2.4.3. Trolox Equivalent Antioxidant Capacity by DPPH Assay
2.4.4. Photochemiluminescence Assay
2.5. Evaluation of Inhibiting Activity Against AGEs
2.6. Statistical Analysis
3. Results and Discussion
3.1. Proximal Chemical Composition and Energy Value of Experimental Gluten-Free Breads
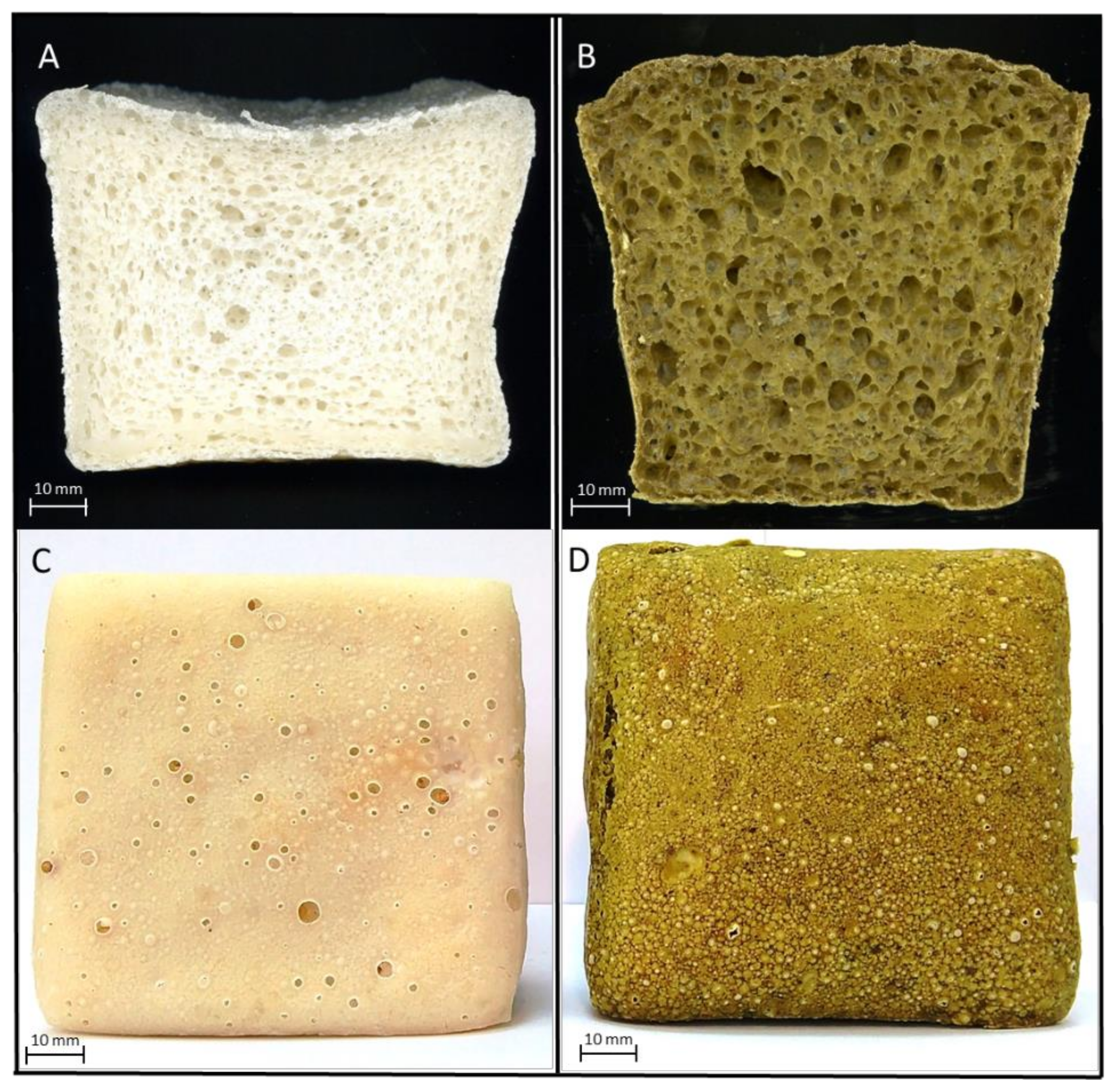
3.2. Technological Parameters of Experimental Gluten-Free Bread
3.3. Textural Properties of Fresh and Stored Experimental Gluten-Free Bread
3.4. Antioxidant Capacity of Experimental Gluten-Free Bread
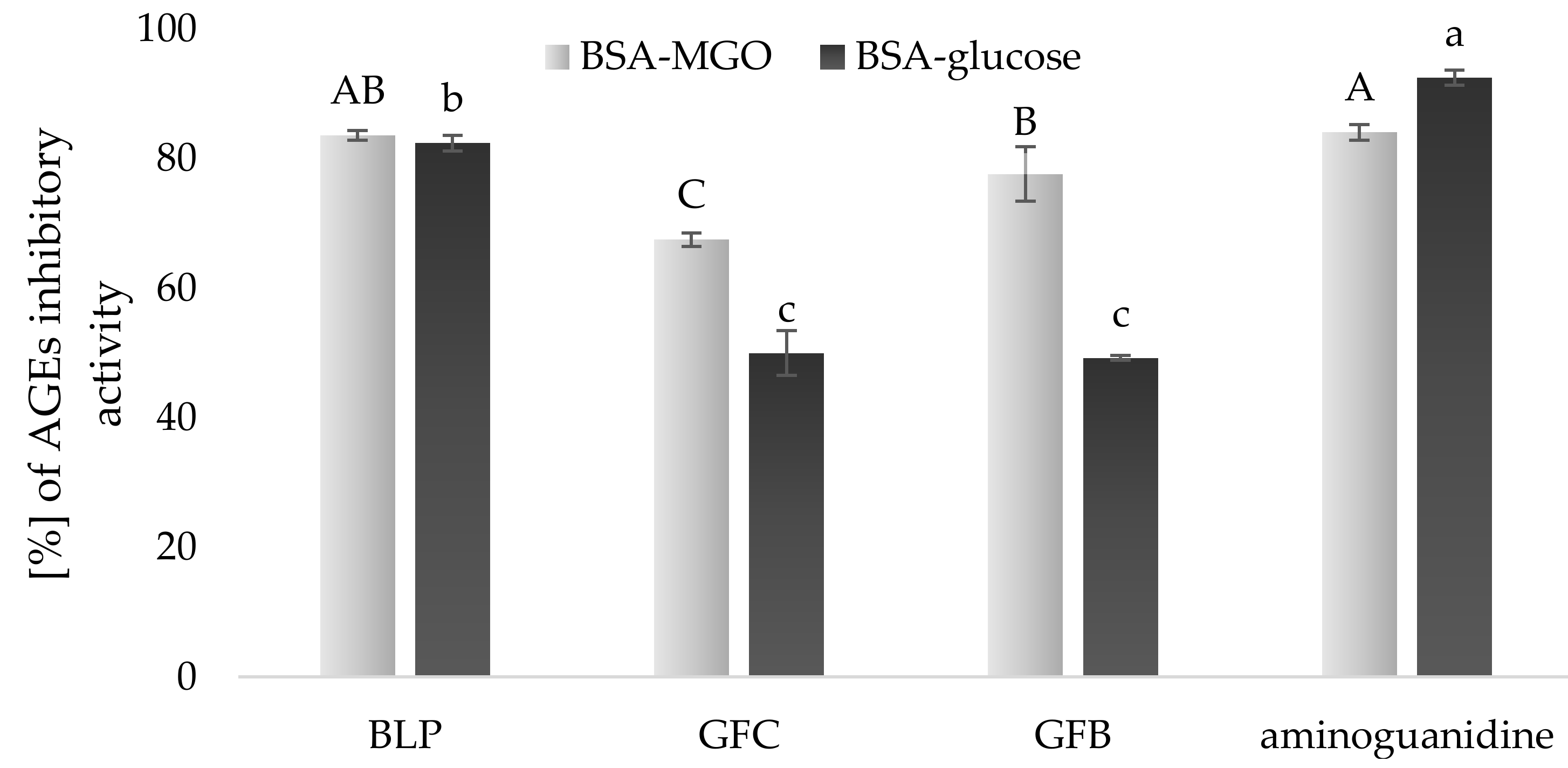
3.5. Anti-AGEs Activity of Experimental Gluten-Free Bread
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Conte, P.; Fadda, C.; Piga, A.; Collar, C. Techno-functional and nutritional performance of commercial breads available in Europe. Food Sci. Technol. Int. 2016, 22, 621–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabanillas, B. Gluten-related disorders: Celiac disease, wheat allergy, and nonceliac gluten sensitivity. Crit. Rev. Food Sci. Nutr. 2020, 60, 2606–2621. [Google Scholar] [CrossRef]
- Matos, M.E.; Rosell, C.M. Understanding gluten-free dough for reaching breads with physical quality and nutritional balance. J. Sci. Food Agric. 2014, 95, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Ronda, F.; Pérez-Quirce, S.; Villanueva, M. Rheological Properties of Gluten-Free Bread Doughs: Relationship with Bread Quality. In Advances in Food Rheology and Its Applications; Woodhead Publishing: Cambridge, UK, 2016; pp. 297–334. [Google Scholar]
- Zannini, E.; Jones, J.M.; Renzetti, S.; Arendt, E.K. Functional Replacements for Gluten. Annu. Rev. Food Sci. Technol. 2012, 3, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Conte, P.; Fadda, C.; Drabińska, N.; Krupa-Kozak, U. Technological and nutritional challenges, and novelty in gluten-free breadmaking: A review. Pol. J. Food Nutr. Sci. 2019, 69. [Google Scholar] [CrossRef]
- Sedlar, T.; Čakarević, J.; Tomić, J.; Popović, L. Vegetable By-Products as New Sources of Functional Proteins. Plant Foods Hum. Nutr. 2020, 76, 31–36. [Google Scholar] [CrossRef]
- Hooper, L.; Cassidy, A. A review of the health care potential of bioactive compounds. J. Sci. Food Agric. 2006, 86, 1805–1813. [Google Scholar] [CrossRef]
- Shapiro, T.A.; Fahey, J.W.; Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Stephenson, K.K.; Wade, K.L.; Ye, L.; Talalay, P. Safety, Tolerance, and Metabolism of Broccoli Sprout Glucosinolates and Isothiocyanates: A Clinical Phase I Study. Nutr. Cancer 2006, 55, 53–62. [Google Scholar] [CrossRef]
- Koch, W. Dietary Polyphenols-Important Non-Nutrients in the Prevention of Chronic Noncommunicable Diseases. A Systematic Review. Nutrients 2019, 11, 1039. [Google Scholar] [CrossRef] [Green Version]
- Korus, J.; Juszczak, L.; Ziobro, R.; Witczak, M.; Grzelak, K.; Sójka, M. Defatted strawberry and blackcurrant seeds as functional ingredients of gluten-free bread. J. Texture Stud. 2012, 43, 29–39. [Google Scholar] [CrossRef]
- Majzoobi, M.; Poor, Z.V.; Jamalian, J.; Farahnaky, A. Improvement of the quality of gluten-free sponge cake using different levels and particle sizes of carrot pomace powder. Int. J. Food Sci. Technol. 2016, 51, 1369–1377. [Google Scholar] [CrossRef]
- Talens, C.; Álvarez-Sabatel, S.; Rios, Y.; Rodríguez, R. Effect of a new microwave-dried orange fibre ingredient vs. a commercial citrus fibre on texture and sensory properties of gluten-free muffins. Innov. Food Sci. Emerg. Technol. 2017, 44, 83–88. [Google Scholar] [CrossRef]
- Littardi, P.; Rinaldi, M.; Grimaldi, M.; Cavazza, A.; Chiavaro, E. Effect of Addition of Green Coffee Parchment on Structural, Qualitative and Chemical Properties of Gluten-Free Bread. Foods 2021, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Herr, I.; Büchler, M.W. Dietary constituents of broccoli and other cruciferous vegetables: Implications for prevention and therapy of cancer. Cancer Treat. Rev. 2010, 36, 377–383. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Świeca, M.; Dziki, D.; Sęczyk, Ł.; Złotek, U.; Różyło, R.; Kaszuba, K.; Ryszawy, D.; Czyż, J. Anticancer and Antioxidant Activity of Bread Enriched with Broccoli Sprouts. BioMed Res. Int. 2014, 2014, 608053. [Google Scholar] [CrossRef]
- Nuñez-Gómez, V.; Baenas, N.; Navarro-González, I.; García-Alonso, J.; Moreno, D.A.; González-Barrio, R.; Periago-Castón, M.J. Seasonal Variation of Health-Promoting Bioactives in Broccoli and Methyl-Jasmonate Pre-Harvest Treatments to Enhance Their Contents. Foods 2020, 9, 1371. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Martínez-Ballesta, M.C.; Carvajal, M.; García-Viguera, C.; Moreno, D.A. Broccoli-derived by-products—A promising source of bioactive ingredients. J. Food Sci. 2010, 75, C383–C392. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, L.; Ser, S.L.; Cumming, J.R.; Ku, K.M. Comparative Phytonutrient Analysis of Broccoli By-Products: The Potentials for Broccoli By-Product Utilization. Molecules 2018, 23, 900. [Google Scholar] [CrossRef] [Green Version]
- Borja-Martínez, M.; Lozano-Sánchez, J.; Borrás-Linares, I.; Pedreño, M.A.; Sabater-Jara, A.B. Revalorization of Broccoli By-Products for Cosmetic Uses Using Supercritical Fluid Extraction. Antioxidants 2020, 9, 1195. [Google Scholar] [CrossRef]
- Lafarga, T.; Gallagher, E.; Bademunt, A.; Viñas, I.; Bobo, G.; Villaró, S.; Aguiló-Aguayo, I. Bioaccessibility, physicochemical, sensorial, and nutritional characteristics of bread containing broccoli co-products. J. Food Process. Preserv. 2019, 43, e13861. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F.; Luziatelli, F.; Ruzzi, M. Functional Ingredients from Agri-Food Waste: Effect of Inclusion Thereof on Phenolic Compound Content and Bioaccessibility in Bakery Products. Antioxidants 2020, 9, 1216. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Drabińska, N.; Rosell, C.M.; Fadda, C.; Anders, A.; Jeliński, T.; Ostaszyk, A. Broccoli leaf powder as an attractive by-product ingredient: Effect on batter behaviour, technological properties and sensory quality of gluten-free mini sponge cake. Int. J. Food Sci. Technol. 2019, 54, 1121–1129. [Google Scholar] [CrossRef] [Green Version]
- Drabińska, N.; Ciska, E.; Szmatowicz, B.; Krupa-Kozak, U. Broccoli by-products improve the nutraceutical potential of gluten-free mini sponge cakes. Food Chem. 2018, 267, 170–177. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, Y.; Lagnika, C.; Li, D.; Liu, C.; Jiang, N.; Song, J.; Zhang, M. A comparative evaluation of nutritional properties, antioxidant capacity and physical characteristics of cabbage (Brassica oleracea var. Capitate var L.) subjected to different drying methods. Food Chem. 2020, 309, 124935. [Google Scholar] [CrossRef] [PubMed]
- Krupa-Kozak, U.; Troszyńska, A.; Bączek, N.; Soral-Śmietana, M. Effect of organic calcium supplements on the technological characteristic and sensory properties of gluten-free bread. Eur. Food Res. Technol. 2011, 232, 497–508. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Current through Revision 4; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2011; pp. 54, 57–58. [Google Scholar]
- FAO. Food Energy—Methods of Analysis and Conversion Factors. Report of a Technical Workshop, Rome, Italy, 3–6 December 2002; FAO Food and Nutrition Paper 77; Food and Agriculture Organization of The United Nations: Rome, Italy, 2003; Available online: http://www.fao.org/3/y5022e/y5022e00.htm#Contents (accessed on 9 April 2021).
- Bourne, M.C.; Kenny, J.F.; Barnard, J. Computer-Assisted Readout Of Data From Texture Profile Analysis Curves1. J. Texture Stud. 1978, 9, 481–494. [Google Scholar] [CrossRef]
- Horszwald, A.; Andlauer, W. Characterisation of bioactive compounds in berry juices by traditional photometric and modern microplate methods. J. Berry Res. 2011, 1, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Zieliński, H.; Zielińska, D.; Kostyra, H. Antioxidant capacity of a new crispy type food products determined by updated analytical strategies. Food Chem. 2012, 130, 1098–1104. [Google Scholar] [CrossRef]
- Szawara-Nowak, D.; Koutsidis, G.; Wiczkowski, W.; Zieliński, H. Evaluation of the in vitro inhibitory effects of buckwheat enhanced wheat bread extracts on the formation of advanced glycation end-products (AGEs). Lebensm. Wiss. Technol. 2014, 58, 327–334. [Google Scholar] [CrossRef]
- Ranawana, V.; Campbell, F.; Bestwick, C.; Nicol, P.; Milne, L.; Duthie, G.; Raikos, V. Breads Fortified with Freeze-Dried Vegetables: Quality and Nutritional Attributes. Part II: Breads Not Containing Oil as an Ingredient. Foods 2016, 5, 62. [Google Scholar] [CrossRef]
- Betoret, E.; Rosell, C.M. Effect of particle size on functional properties of Brassica napobrassica leaves powder. Starch interactions and processing impact. Food Chem. X 2020, 8, 100106. [Google Scholar] [CrossRef]
- Vidrih, R.; Filip, S.; Hribar, J. Content of higher fatty acids in green vegetables. Czech J. Food Sci. 2009, 27, 125–129. [Google Scholar] [CrossRef]
- Arnáiz, E.; Bernal, J.; Martín, M.T.; García-Viguera, C.; Bernal, J.L.; Toribio, L. Supercritical fluid extraction of lipids from broccoli leaves. Eur. J. Lipid Sci. Technol. 2011, 113, 479–486. [Google Scholar] [CrossRef]
- Ding, S.; Peng, B.; Li, Y.; Yang, J. Evaluation of specific volume, texture, thermal features, water mobility, and inhibitory effect of staling in wheat bread affected by maltitol. Food Chem. 2019, 283, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Rosell, C.M.; Benedito de Barber, C. Effect of the addition of different fibres on wheat dough performance and bread quality. Food Chem. 2002, 79, 221–226. [Google Scholar] [CrossRef]
- Miranda-Ramos, K.C.; Haros, C.M. Combined Effect of Chia, Quinoa and Amaranth Incorporation on the Physico-Chemical, Nutritional and Functional Quality of Fresh Bread. Foods 2020, 9, 1859. [Google Scholar] [CrossRef] [PubMed]
- Fratelli, C.; Muniz, D.G.; Santos, F.G.; Capriles, V.D. Modelling the effects of psyllium and water in gluten-free bread: An approach to improve the bread quality and glycemic response. J. Funct. Foods 2018, 42, 339–345. [Google Scholar] [CrossRef]
- Elgeti, D.; Nordlohne, S.D.; Föste, M.; Besl, M.; Linden, M.H.; Heinz, V.; Jekle, M.; Becker, T. Volume and texture improvement of gluten-free bread using quinoa white flour. J. Cereal Sci. 2014, 59, 41–47. [Google Scholar] [CrossRef]
- Hu, S.H.; Wang, J.C.; Kung, H.F.; Wang, J.T.; Lee, W.L.; Yang, Y.H. Antimicrobial Effect of Extracts of Cruciferous Vegetables. Kaohsiung J. Med. Sci. 2004, 20, 591–599. [Google Scholar] [CrossRef] [Green Version]
- Conte, P.; Del Caro, A.; Balestra, F.; Piga, A.; Fadda, C. Bee pollen as a functional ingredient in gluten-free bread: A physical-chemical, technological and sensory approach. Lebensm. Wiss. Technol. 2018, 90, 1–7. [Google Scholar] [CrossRef]
- Karam, M.C.; Petit, J.; Zimmer, D.; Baudelaire Djantou, E.; Scher, J. Effects of drying and grinding in production of fruit and vegetable powders: A review. J. Food Eng. 2016, 188, 32–49. [Google Scholar] [CrossRef]
- Ranawana, V.; Raikos, V.; Campbell, F.; Bestwick, C.; Nicol, P.; Milne, L.; Duthie, G. Breads Fortified with Freeze-Dried Vegetables: Quality and Nutritional Attributes. Part 1: Breads Containing Oil as an Ingredient. Foods 2016, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.; Gormley, T.R.; Arendt, E.K. Crust and crumb characteristics of gluten free breads. J. Food Eng. 2003, 56, 153–161. [Google Scholar] [CrossRef]
- Belorio, M.; Gómez, M. Effect of Hydration on Gluten-Free Breads Made with Hydroxypropyl Methylcellulose in Comparison with Psyllium and Xanthan Gum. Foods 2020, 9, 1548. [Google Scholar] [CrossRef] [PubMed]
- Sarabhai, S.; Tamilselvan, T.; Prabhasankar, P. Role of enzymes for improvement in gluten-free foxtail millet bread: It’s effect on quality, textural, rheological and pasting properties. Lebensm. Wiss. Technol. 2021, 137, 110365. [Google Scholar] [CrossRef]
- Gray, J.A.; Bemiller, J.N. Bread staling: Molecular basis and control. Compr. Rev. Food Sci. Food Saf. 2003, 2, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.; Abellán-Victorio, A.; Beretta, V.; Camargo, A.; Moreno, D.A. Functional Ingredients from Brassicaceae Species: Overview and Perspectives. Int. J. Mol. Sci. 2020, 21, 1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soengas, P.; Cartea, M.E.; Francisco, M.; Sotelo, T.; Velasco, P. New insights into antioxidant activity of Brassica crops. Food Chem. 2012, 134, 725–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostermann-Porcel, M.V.; Quiroga-Panelo, N.; Rinaldoni, A.N.; Campderrós, M.E. Incorporation of Okara into Gluten-Free Cookies with High Quality and Nutritional Value. J. Food Qual. 2017, 2017, 4071585. [Google Scholar] [CrossRef] [Green Version]
- Ames, J.M. Evidence against dietary advanced glycation endproducts being a risk to human health. Mol. Nutr. Food Res. 2007, 51, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; del Castillo, M.D.; de la Maza, M.P.; Filip, R.; Gugliucci, A.; Luevano-Contreras, C.; Macías-Cervantes, M.H.; Markowicz Bastos, D.H.; Medrano, A.; Menini, T.; et al. Dietary advanced glycation end products and their role in health and disease. Adv. Nutr. 2015, 6, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Sotokawauchi, A.; Ishibashi, Y.; Matsui, T.; Yamagishi, S.I. Aqueous Extract of Glucoraphanin-Rich Broccoli Sprouts Inhibits Formation of Advanced Glycation End Products and Attenuates Inflammatory Reactions in Endothelial Cells. Evid. Based Complement. Altern. Med. 2018, 2018, 9823141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, X.; Ma, J.; Cheng, K.W.; Jiang, Y.; Chen, F.; Wang, M. The effects of grape seed extract fortification on the antioxidant activity and quality attributes of bread. Food Chem. 2010, 119, 49–53. [Google Scholar] [CrossRef]
| Ingredient (%) | GFC | GFB |
|---|---|---|
| Corn starch | 36.7 | 31.7 |
| Potato starch | 8.9 | 8.9 |
| Pectin | 2.2 | 2.2 |
| Sugar | 2.8 | 2.8 |
| Salt | 0.8 | 0.8 |
| Oil | 1.4 | 1.4 |
| Fresh yeast | 2.8 | 2.8 |
| BLP | - | 5 |
| Water | 44.4 | 44.4 |
| GFC | GFB | p-Value | |
|---|---|---|---|
| Moisture | 55.67 a ± 0.18 | 55.32 a ± 0.15 | 0.0628 |
| Proteins | 1.22 b ± 0.04 | 2.38 a ± 0.09 | 0.0004 |
| Ash | 1.81 b ± 0.03 | 2.16 a ± 0.04 | 0.0004 |
| Fat | 0.87 b ± 0.01 | 2.33 a ± 0.03 | <0.0001 |
| Carbohydrates * | 40.56 a ± 0.15 | 37.81 b ± 0.08 | <0.0001 |
| Energy value (kJ) | 740 b ± 4 | 769 a ± 2 | 0.0060 |
| Energy value (kcal) | 177 b ± 1 | 184 a ± 1 | 0.0010 |
| GFC | GFB | p Value | |
|---|---|---|---|
| Specific volume (mL/g) | 2.41 b ± 0.14 | 3.08 a ± 0.16 | 0.0058 |
| Bake loss (%) | 14.96 b ± 0.09 | 12.07 a ± 0.63 | 0.0141 |
| Crust colour | |||
| L* | 75.89 a ± 1.70 | 50.41 b ± 1.52 | <0.001 |
| a* | 1.58 b ± 0.08 | −3.65 a ± 0.31 | <0.001 |
| b* | 17.28 b ± 1.12 | 31.95 a ± 0.94 | <0.001 |
| Crumb colour | |||
| L* | 71.58 a ± 1.70 | 34.92 b ± 2.81 | <0.001 |
| a* | 0.35 b ± 0.11 | −1.47 a ± 0.14 | <0.001 |
| b* | 11.15 b ± 0.73 | 27.93 a ± 1.85 | <0.001 |
| GFC | GFB | p-Value | |
|---|---|---|---|
| Hardness (N) | |||
| Fresh | 13.21 aC ± 1.22 | 13.80 aC ± 0.07 | 0.4905 |
| Stored 24 h | 29.53 aB ± 4.67 | 33.16 aB ± 4.63 | 0.3932 |
| Stored 72 h | 45.78 aA ± 2.55 | 42.86 aA ± 4.67 | 0.2427 |
| Springiness | |||
| Fresh | 0.93 bA ± 0.02 | 0.99 aA ± 0.01 | 0.0196 |
| Stored 24 h | 0.90 aA ± 0.08 | 0.92 aB ± 0.03 | 0.7167 |
| Stored 72 h | 0.83 aA ± 0.01 | 0.89 aC ± 0.03 | 0.0620 |
| Cohesiveness | |||
| Fresh | 0.55 bA ± 0.07 | 0.77 aA ± 0.02 | 0.0249 |
| Stored 24 h | 0.34 aB ± 0.11 | 0.44 aB ± 0.03 | 0.2523 |
| Stored 72 h | 0.28 aC ± 0.01 | 0.30 aC ± 0.01 | 0.0705 |
| Chewiness | |||
| Fresh | 6.73 bB ± 1.53 | 10.45 aA ± 0.21 | 0.0496 |
| Stored 24 h | 8.88 aB ± 2.09 | 13.51 aA ± 2.53 | 0.0733 |
| Stored 72 h | 10.77 aA ± 0.91 | 11.51 aA ± 1.10 | 0.4217 |
| Resilience | |||
| Fresh | 0.31 aA ± 0.08 | 0.50 aA ± 0.01 | 0.0523 |
| Stored 24 h | 0.16 aB ± 0.07 | 0.24 aB ± 0.01 | 0.1841 |
| Stored 72 h | 0.12 aC ± 0.02 | 0.13 aC ± 0.01 | 0.4961 |
| BLP | GFC | GFB | p Value | |
|---|---|---|---|---|
| TFC (mg GAE/g dm) | 14.42 ± 0.18 | 0.64 b ± 0.04 | 1.25 a ± 0.05 | 0.001 |
| ACW (µmol/g dm) | 3.29 ± 0.10 | 0.03 b ± 0.01 | 1.64 a ± 0.08 | 0.007 |
| ACL (µmol/g dm) | 1191.25 ± 64.37 | 1.05 b ± 0.04 | 106.97 a ± 0.87 | <0.001 |
| ABTS (µmol TE/g dm) | 34.33 ± 0.29 | 0.13 b ± 0.01 | 1.77 a ± 0.06 | 0.003 |
| DPPH (µmol TE/g dm) | 34.11 ± 0.29 | 0.27 b ± 0.03 | 0.95 a ± 0.05 | 0.001 |
| BSA-MGO | BSA-Glucose | |
|---|---|---|
| ACW | 0.988 | 0.859 |
| ACL | 0.829 | 0.995 |
| ABTS | 0.808 | 0.998 |
| DPPH | 0.793 | 0.999 |
| TPC | 0.806 | 0.998 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupa-Kozak, U.; Drabińska, N.; Bączek, N.; Šimková, K.; Starowicz, M.; Jeliński, T. Application of Broccoli Leaf Powder in Gluten-Free Bread: An Innovative Approach to Improve Its Bioactive Potential and Technological Quality. Foods 2021, 10, 819. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10040819
Krupa-Kozak U, Drabińska N, Bączek N, Šimková K, Starowicz M, Jeliński T. Application of Broccoli Leaf Powder in Gluten-Free Bread: An Innovative Approach to Improve Its Bioactive Potential and Technological Quality. Foods. 2021; 10(4):819. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10040819
Chicago/Turabian StyleKrupa-Kozak, Urszula, Natalia Drabińska, Natalia Bączek, Kristýna Šimková, Małgorzata Starowicz, and Tomasz Jeliński. 2021. "Application of Broccoli Leaf Powder in Gluten-Free Bread: An Innovative Approach to Improve Its Bioactive Potential and Technological Quality" Foods 10, no. 4: 819. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10040819









