Food Ingredients and Nutraceuticals from Microalgae: Main Product Classes and Biotechnological Production
Abstract
:1. Introduction
2. Microalgae Production
2.1. Microalgae Cultivation
2.1.1. Illumination
2.1.2. Diverse Growth Conditions
2.2. Cultivation Systems
2.2.1. Open Ponds
2.2.2. Closed Photobioreactors
2.3. Contaminations
2.4. Downstream Processing
3. Rentability of Microalgae Production
4. Nutritional Composition of Microalgae
5. Food Ingredients and Nutraceuticals from Microalgae
5.1. Microalgae Used as a Food Ingredient
5.1.1. Addition of Microalgae to Biscuits
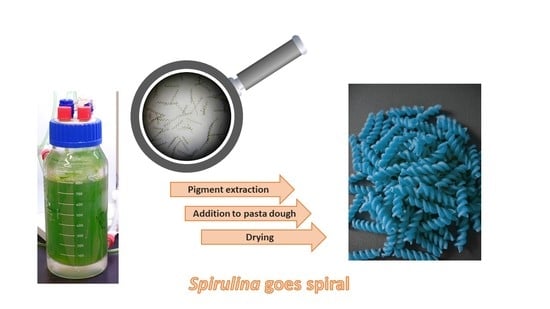
5.1.2. Addition of Microalgae to Pasta and Noodles
5.2. Isolated Products from Microalgae for Enrichment of Foods
5.2.1. n-3 Fatty Acids
5.2.2. Carotene and Other Carotenoids
5.3. Microalgae as Food Supplements
5.4. Toxicological Relevance of Microalgal Products
6. Conclusions
- New hygienic cultivation systems for the production of pure cultures under controlled conditions to achieve high cell densities. High product concentrations (i.e., biomass, fat, secondary metabolites) are a prerequisite for industrial production. Improved cultivation systems facilitate the industrial production of selected species (Arthrospira platensis, Chlorella vulgaris) with high nutritional values (high protein contents) and interesting metabolites (food colorants, antioxidants etc.).
- Consumers demand vegan foods, natural foods, and sustainable food production. Microalgae has become a ‘fashion nutraceutical’ in Western countries.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Habib, M.A.B.; Parvin, M.; Huntington, T.C.; Hasan, M.R. A Review on Culture, Production and Use of Spirulina as Food for Humans and Feeds for Domestic Animals and Fish; FAO Food and Agriculture Organization of the United Nations: Rome, Italy, 2008. [Google Scholar]
- Dolganyuk, V.; Andreeva, A.; Budenkova, E.; Sukhikh, S.; Babich, O.; Ivanova, S.; Prosekov, A.; Ulrikh, E. Study of morphological features and determination of the fatty acid composition of the microalgae lipid complex. Biomolecules 2020, 10, 1571. [Google Scholar] [CrossRef] [PubMed]
- Katircioglu, H.; Akin, B.S.; Atici, T. Microalgal toxin(s): Characteristics and importance. Afr. J. Biotechnol. 2004, 3, 667–674. [Google Scholar]
- Karnaouri, A.; Chalima, A.; Kalogiannis, K.G.; Varamogianni-Mamatsi, D.; Lappas, A.; Topakas, E. Utilization of lignocellulosic biomass towards the production of omega-3 fatty acids by the heterotrophic marine microalga Crypthecodinium cohnii. Biores. Technol. 2020, 303, 122899. [Google Scholar] [CrossRef]
- Patil, V.; Källqvist, T.; Olsen, E.; Vogt, G.; Gislerød, H.R. Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquacult. Int. 2007, 15, 1–9. [Google Scholar] [CrossRef]
- Bhattacharjya, R.; Marella, T.K.; Tiwari, A.; Saxena, A.; Singh, P.K.; Mishra, B. Bioprospecting of marine diatoms Thalassiosira, Skeletonema and Chaetoceros for lipids and other value-added products. Biores. Technol. 2020, 318, 24073. [Google Scholar] [CrossRef] [PubMed]
- Koller, M. Design of closed photobioreactors for algal cultivation. In Algal Biorefineries; Prokop, A., Bajpai, R.K., Zappi, M.E., Eds.; Springer: Heidelberg, Germany, 2015; pp. 133–186. [Google Scholar]
- Huang, Q.; Jiang, F.; Wang, L.; Yang, C. Design of photobioreactors for mass cultivation of photosynthetic organisms. Engineering 2017, 3, 318–329. [Google Scholar] [CrossRef]
- Han, D.; Li, Y.; Hu, Q. Astaxanthin in microalgae: Pathways, functions and biotechnological implications. Algae 2013, 28, 131–147. [Google Scholar] [CrossRef]
- Mulders, K.J.M.; Lamers, P.P.; Martens, D.E.; Wijffels, R.H. Phototrophic pigment production with microalgae: Biological constraints and opportunities. J. Phycol. 2014, 50, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Morales-Sánchez, D.; Martinez-Rodriguez, O.A.; Martinez, A. Heterotrophic cultivation of microalgae: Production of metabolites of commercial interest. J. Chem. Technol. Biotechnol. 2017, 92, 925–936. [Google Scholar] [CrossRef]
- Hu, J.; Nagarajan, D.; Zhang, Q.; Chang, J.-S.; Lee, D.J. Heterotrophic cultivation of microalgae for pigment production: A review. Biotechnol. Adv. 2018, 36, 54–67. [Google Scholar] [CrossRef]
- Daneshvar, E.; Ok, Y.S.; Tavakoli, S.; Sarkar, B.; Shaheen, S.M.; Hong, H.; Luo, Y.; Rinklebe, J.; Song, H.; Bhatnagar, A. Insight into upstream processing of microalgae: A review. Biores. Technol. 2021, 329, 124870. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zheng, Y.; Yu, L.; Chen, S. Mixotrophic cultivation of a Chlorella sorokiniana strain for enhanced biomass and lipid production. Biomass Bioenergy 2014, 66, 204–213. [Google Scholar] [CrossRef]
- Wen, X.; Wang, Z.; Ding, Y.; Geng, Y.; Li, Y. Enhancing the production of astaxanthin by mixotrophic cultivation of Haematococcus pluvialis in open raceway ponds. Aquac. Int. 2020, 28, 625–638. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Guerra, I.; Pereira, H.; Costa, M.; Silva, J.T.; Santos, T.; Varela, J.; Mateus, M.; Silva, J. Operation regimes: A comparison based on Nannochloropsis oceanica biomass and lipid productivity. Energies 2021, 14, 1542. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Meireles, L.A.; Malcata, F.X. Microalgal reactors: A review of enclosed system designs and performances. Biotechnol. Prog. 2006, 22, 1490–1506. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.J.; Harrison, S.T.L. Aeration energy requirements for lipid production by Scenedesmus sp. in airlift bioreactors. Algal Res. 2014, 5, 249–257. [Google Scholar] [CrossRef]
- Ger, K.A.; Urrutia-Corderob, P.; Frost, P.C.; Hansson, L.-A.; Sarnelle, O.; Wilson, A.E.; Lürling, M. The interaction between cyanobacteria and zooplankton in a more eutrophic world. Harmful Algae 2016, 54, 128–144. [Google Scholar] [CrossRef]
- Jang, M.-H.; Ha, K.; Joo, G.-J.; Takamura, N. Toxin production of cyanobacteria is increased by exposure to zooplankton. Freshw. Biol. 2003, 48, 1540–1550. [Google Scholar] [CrossRef]
- Karuppasamy, S.; Musale, A.S.; Soni, S.; Bhadra, B.; Gujarathi, N.; Sundaram, M.; Sapre, A.; Dasgupta, S.; Kumar, C. Integrated grazer management mediated by chemicals for sustainable cultivation of algae in open ponds. Algal Res. 2018, 35, 39–448. [Google Scholar] [CrossRef]
- Richmond, G. Biological principles of mass cultivation of photoautotrophic microalgae. In Handbook of Microalgal Culture, 2nd ed.; Richmond, A., Hu, Q., Eds.; Wiley: Chichester, UK, 2013; pp. 169–204. [Google Scholar]
- Koller, M.; Salerno, A.; Tuffner, P.; Koinigg, M.; Böchzelt, H.; Schober, S.; Pieber, S.; Schnitzer, H.; Mittelbach, M.; Braunegg, G. Characteristics and potential of micro algal cultivation strategies: A review. J. Clean Prod. 2012, 37, 377–388. [Google Scholar] [CrossRef]
- Day, J.; Thomas, J.; Achilles-Day, U.E.; Leakey, R.J. Early detection of protozoan grazers in algal biofuel cultures. Biores. Technol. 2012, 114, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Tejido-Nuñez, Y.; Aymerich, E.; Sancho, L.; Refardt, D. Co-cultivation of microalgae in aquaculture water: Interactions, growth and nutrient removal efficiency at laboratory- and pilot-scale. Algal Res. 2020, 49, 101940. [Google Scholar] [CrossRef]
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C.M. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef] [Green Version]
- Grima, E.M.; Fernández, F.G.A.; Robles-Medina, A. Downstream processing of cell mass and products. In Handbook of Microalgal Culture, 2nd ed.; Richmond, A., Hu, Q., Eds.; Wiley: Chichester, UK, 2013; pp. 267–309. [Google Scholar]
- González López, C.V.; Acién Fernández, F.G.; Fernández Sevilla, J.M.; Sánchez Fernández, J.F.; Cerón García, M.C.; Molina Grima, E. Utilization of the cyanobacteria Anabaena sp. ATCC 33047 in CO2 removal processes. Biores. Technol. 2009, 100, 5904–5910. [Google Scholar] [CrossRef]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Monte, J.; Sá, M.; Galinha, C.F.; Costa, L.; Hoekstrad, H.; Brazinha, C.; Crespo, J.G. Harvesting of Dunaliella salina by membrane filtration at pilot scale. Sep. Purif. Technol. 2018, 190, 252–260. [Google Scholar] [CrossRef]
- Desmorieux, H.; Hernandez, F. Biochemical and physical criteria of Spirulina after different drying processes. In Proceedings of the 14th International Drying Symposium (IDS), São Paulo City, Brazil, 22–25 August 2004; pp. 900–907. [Google Scholar]
- Walsh, M.J.; Van Doren, L.G.; Shete, N.; Prakash, A.; Salim, U. Financial tradeoffs of energy and food uses of algal biomass under stochastic conditions. Appl. Energy 2018, 210, 591–603. [Google Scholar] [CrossRef]
- Nova, P.; Martins, A.P.; Teixeira, C.; Abreu, H.; Silva, J.G.; Machado Silva, A.; Freitas, A.C.; Gomes, A.M. Foods with microalgae and seaweeds fostering consumers health: A review on scientific and market innovations. J. Appl. Phycol. 2020, 32, 1789–1802. [Google Scholar] [CrossRef]
- Enzing, C.; Ploeg, M.; Barbosa, M.; Sijtsma, L. Microalgae-based products for the food and feed sector: An outlook for Europe. In EUR—Scientific and Technical Research Series; Vigani, M., Parisi, C., Cerezo, E.R., Eds.; European Commission, EUR 26255—Joint Research Centre—Institute for Prospective Technological Studies, Publications Office: Luxembourg, 2014. [Google Scholar]
- Amorim, M.L.; Soares, J.; dos Reis Coimbra, J.S.; de Oliveira Leite, M.; Teixeira Albino, L.F.; Arêdes Martins, M. Microalgae proteins: Production, separation, isolation, quantification, and application in food and feed. Crit. Rev. Food Sci. Nutr. 2020, 61, 1976–2002. [Google Scholar] [CrossRef]
- Grossman, A. Nutrient acquisition: The generation of bioactive vitamin B12 by microalgae. Curr. Biol. 2016, 26, R319–R337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, W.; Clarke, W.; Pratt, S. Cycling of iodine by microalgae: Iodine uptake and release by a microalgae biofilm in a groundwater holding pond. Ecol. Engin. 2016, 94, 286–294. [Google Scholar] [CrossRef]
- Iwamoto, K.; Shiraiwa, Y. Characterization of intracellular iodine accumulation by iodine-tolerant microalgae. Procedia Environm. Sci. 2012, 15, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Müssig, K. Chapter 93—Iodine-induced toxic effects due to seaweed consumption. In Comprehensive Handbook of Iodine; Preedy, V.R., Burrow, G.N., Watson, R., Eds.; Academic Press: Amsterdam, The Netherlands, 2009; pp. 897–908. [Google Scholar]
- Begum, H.; Yusoff, F.M.D.; Banerjee, S.; Khatoon, H.; Shariff, M. Availability and utilization of pigments from microalgae. Crit. Rev. Food Sci. Nutr. 2016, 56, 2209–2222. [Google Scholar] [CrossRef] [PubMed]
- Kartik, A.; Akhil, D.; Lakshmi, D.; Gopinath, K.P.; Arun, J.; Sivaramakrishnan, R.; Pugazhendhi, A. A critical review on production of biopolymers from algae biomass and their applications. Biores. Technol. 2021, 329, 124868. [Google Scholar] [CrossRef] [PubMed]
- Chacon-Lee, T.L.; Gonzalez-Marino, G.E. Microalgae for “healthy” foods—Possibilities and challenges. Compr. Rev. Food Sci. Food Saf. 2010, 9, 655–675. [Google Scholar] [CrossRef]
- FAO/WHO. Energy and protein requirement. In Report of a Joint FAO/WHO Ad Hoc Expert Committee; FAO: Geneva, Switzerland, 1973; Volume 52. [Google Scholar]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Sandgruber, F.; Gielsdorf, A.; Baur, A.C.; Schenz, B.; Müller, S.M.; Schwerdtle, T.; Stangl, G.I.; Griehl, C.; Lorkowski, S.; Dawczynski, C. Variability in macro- and micronutrients of 15 commercially available microalgae powders. Mar. Drugs 2021, 19, 310. [Google Scholar] [CrossRef]
- Lafarga, T. Effect of microalgal biomass incorporation into foods: Nutritional and sensorial attributes of the end products. Algal Res. 2019, 41, 101566. [Google Scholar] [CrossRef]
- da Silva, S.P.; Ferreira do Valle, A.; Perrone, D. Microencapsulated Spirulina maxima biomass as an ingredient for the production of nutritionally enriched and sensorially well-accepted vegan biscuits. LWT Food Sci. Technol. 2021, 142, 110997. [Google Scholar] [CrossRef]
- Mantecón, L.; Moyano, R.; Cameánc, A.M.; Jos, A. Safety assessment of a lyophilized biomass of Tetraselmis chuii (TetraSOD®) in a 90 day feeding study. Food Chem. Toxicol. 2019, 133, 110810. [Google Scholar] [CrossRef]
- Plankton Marino. Available online: https://www.planctonmarino.com (accessed on 28 May 2021).
- Bernaerts, T.M.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. The potential of microalgae and their biopolymers as structuring ingredients in food: A review. Biotechnol. Adv. 2019, 37, 107419. [Google Scholar] [CrossRef] [PubMed]
- Sahni, P.; Sharma, S.; Singh, B. Evaluation and quality assessment of defatted microalgae meal of Chlorella as an alternative food ingredient in cookies. Nutr. Food Sci. 2019, 49, 221–231. [Google Scholar] [CrossRef]
- Onacik-Gür, S.; Zbikowska, A.; Majewska, B. Effect of Spirulina (Spirulina platensis) addition on textural and quality properties of cookies. Ital. J. Food Sci. 2017, 30, 1–12. (In Italian) [Google Scholar]
- Kumoro, A.C.; Johnny, D.; Alfilovita, D. Incorporation of microalgae and seaweed in instant fried wheat noodles manufacturing: Nutrition and culinary properties study. Int. Food Res. J. 2016, 23, 715–722. [Google Scholar]
- de Marco, E.R.; Steffolani, M.E.; Martínez, C.S.; León, C.S. Effects of spirulina biomass on the technological and nutritional quality of bread wheat pasta. LWT Food Sci. Technol. 2014, 58, 102–108. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Abdo, S.M.; Hussein, A.M.S. Microalgae Dunaliella salina for use as food supplement to improve pasta quality. Int. J. Pharm. Sci. Rev. Res. 2017, 46, 45–51. [Google Scholar]
- Fradique, M.; Batista, A.P.; Nunes, M.C.; Gouveia, L.; Bandarra, N.M.; Raymundo, A. Incorporation of Chlorella vulgaris and Spirulina maxima biomass in pasta products. Part 1: Preparation and evaluation. J. Sci. Food Agric. 2010, 90, 1656–1664. [Google Scholar] [CrossRef]
- Buono, S.; Langellotti, A.L.; Martello, A.; Rinna, F.; Fogliano, V. Functional ingredients from microalgae. Food Funct. 2014, 5, 1669–1685. [Google Scholar] [CrossRef]
- Fradique, M.; Batista, A.P.; Nunes, M.C.; Gouveia, L.; Bandarra, N.M.; Raymundo, A. Isochrysis galbana and Diacronema vlkianum biomass incorporation in pasta products as PUFA’s source. LWT Food Sci. Technol. 2013, 50, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Böhm, T.; Berger, H.; Nejabat, M.; Riegler, T.; Kellner, F.; Kuttke, M.; Sagmeister, S.; Bazanella, M.; Stolze, K.; Daryabeigi, A.; et al. Food-derived peroxidized fatty acids may trigger hepatic inflammation: A novel hypothesis to explain steatohepatitis. J. Hepatol. 2013, 59, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Ryckebosch, E.; Bruneel, C.; Termote-Verhalle, R.; Goiris, K.; Muylaert, K.; Foubert, I. Nutritional evaluation of microalgae oils rich in omega-3 long chain polyunsaturated fatty acids as an alternative for fish oil. Food Chem. 2014, 160, 393–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemahieu, C.; Bruneel, C.; Ryckebosch, E.; Muylaert, K.; Buyse, J.; Foubert, I. Impact of different omega-3 polyunsaturated fatty acid (n-3 PUFA) sources (flaxseed, Isochrysis galbana, fish oil and DHA Gold) on n-3 LC-PUFA enrichment (efficiency) in the egg yolk. J. Funct. Foods 2015, 19, 821–827. [Google Scholar] [CrossRef]
- Shen, Y.; Guo, C.; Lu, T.; Ding, X.-Y.; Zhao, M.-T.; Zhang, M.; Liu, H.-L.; Song, L.; Zhou, D.-Y. Effects of gallic acid alkyl esters and their combinations with other antioxidants on oxidative stability of DHA algae oil. Food Res. Int. 2021, 143, 110280. [Google Scholar] [CrossRef] [PubMed]
- de Jesus Raposo, M.F.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Carotenoids from marine microalgae: A valuable natural source for the prevention of chronic diseases. Mar. Drugs 2015, 13, 5128–5155. [Google Scholar] [CrossRef] [PubMed]
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens); Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Scientifc Opinion on the safety of astaxanthin for its use as a novel food in food supplements. EFSA J. 2020, 18, 5993. [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens); Turck, D.; EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific opinion on the re-evaluation of mixed carotenes (E 160a(i)) and beta-carotene (E 160a (ii)) as a food additive. EFSA J. 2012, 10, 2593. [Google Scholar]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the sea: Algae as source of bioactive carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef] [PubMed]
- Fen, L.; Nie, K.; Jiang, H.; Fan, W. Effects of lutein supplementation in age-related macular degeneration. PLoS ONE 2019, 14, e0227048. [Google Scholar]
- Plaza, M.; Cifuentes, A.; Ibanez, E. In the search of new functional food ingredients from algae. Trends Food Sci. Technol. 2008, 19, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672. [Google Scholar] [CrossRef] [PubMed]
- Boussiba, S.; Vonshak, A. Astaxanthin accumulation in the green alga Haematococcus pluvialis. Plant Cell Physiol. 1991, 32, 1077–1082. [Google Scholar] [CrossRef] [Green Version]
- Heussner, A.H.; Mazija, L.; Fastner, J.; Dietrich, D.R. Toxin content and cytotoxicity of algal dietary supplements. Toxicol. Appl. Pharmacol. 2012, 265, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, J.L.; de Vicente, M.; Galán, B. Microalgae, old sustainable food and fashion nutraceuticals. Microb. Biotechnol. 2017, 10, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- Eltanahy, E.; Torky, A. Microalgae as cell factories: Food and feed-grade high-value metabolites. In Microalgal Biotechnology: Recent Advances, Market Potential, and Sustainability; Royal Society of Chemistry: Cambridge, UK, 2021; pp. 1–35. [Google Scholar]



| Parameter | Open Ponds | Raceway Ponds | Bubble Columns | Tube Reactors | STR PBR |
|---|---|---|---|---|---|
| Mixing | very low | low | medium | medium | high |
| Illumination | very low | low | medium | high | medium |
| Sterility | no | low | possible | possible | yes |
| Capital and operational cost | very low | very low | medium | medium | high |
| Species | Protein (wt%) | Carbohydrate (wt%) | Lipid (wt%) |
|---|---|---|---|
| Nannochloropsis sp. | 29–32 | 9–36 | 15–18 |
| Nannochloropsis oceanica | 29 | 32–39 | 19–24 |
| Botryococcus braunii | 70 | – | – |
| Arthrospira platensis | 53–70 | 12–24 | 6–20 |
| Chlorella vulgaris | 49–55 | 7–42 | 3–36 |
| Haematococcus pluvialis | 48 | 27 | 15 |
| Isochrysis galbana | 27 | 17 | 17 |
| Dunaliella salina | 57 | 32 | 6 |
| Scenedesmus obliquus | 50–56 | 10–17 | 12–14 |
| Porphyridium cruentum | 28–39 | 40–57 | 9–14 |
| Species | LA | ALA | GLA | STA | AA | EPA | DPA | DHA |
|---|---|---|---|---|---|---|---|---|
| Chlorella vulgaris | 3–10 | 2–21 | 15–24 | Traces | Traces | 3 | 3 | 21 |
| Arthrospira platensis | 10–21 | 1 | 1–25 | 1 | 0.3–0.4 | 2–3 | - | 3 |
| Isochrysis galbana | 1 | 0.5 | 0.5 | 1 | 1 | 2 | Traces | 19 |
| Scenedesmus obliquus | 1–2 | 4–22 | 0.1–4 | 1–3 | 0.1–0.2 | - | - | - |
| Haematococcus pluvialis | 4 | 15 | 0.1 | - | - | - | - | - |
| Porphyridium cruentum | 0.4–25 | - | - | - | 1–35 | 1–27 | - | 6.1 |
| Nannochloropsis oceanica | 0.6–0.8 | - | - | - | 1.4–1.9 | 8.4–11 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kratzer, R.; Murkovic, M. Food Ingredients and Nutraceuticals from Microalgae: Main Product Classes and Biotechnological Production. Foods 2021, 10, 1626. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10071626
Kratzer R, Murkovic M. Food Ingredients and Nutraceuticals from Microalgae: Main Product Classes and Biotechnological Production. Foods. 2021; 10(7):1626. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10071626
Chicago/Turabian StyleKratzer, Regina, and Michael Murkovic. 2021. "Food Ingredients and Nutraceuticals from Microalgae: Main Product Classes and Biotechnological Production" Foods 10, no. 7: 1626. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10071626