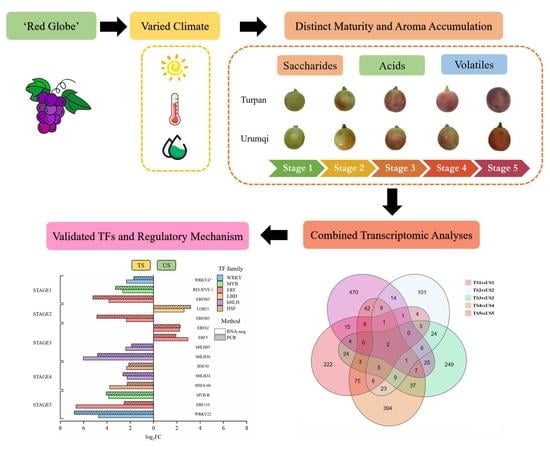
Effect of Climate on Volatile Metabolism in ‘Red Globe’ Grapes (Vitis vinifera L.) during Fruit Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Growth Environment
2.2. Determinations of Saccharides and Organic Acids
2.3. Volatile Analysis
2.4. RNA Sequencing
2.5. The Validation of TFs
2.6. Weighted Gene Co-Expression Network Analysis (WGCNA)
2.7. Statistical Analysis
3. Results
3.1. Saccharides and Organic Acids
3.2. Volatile Profiles
3.3. Transcriptomic and TF Analyses
3.4. Verification of TFs
4. Discussion
4.1. Saccharides and Organic Acids Indicated Grape Maturity
4.2. Climate and Fruit Growth Resulted in the Discrepancies in Volatiles in Grapes
4.3. Principal Genes and TFs
4.4. WGCNA Analyses on Volatiles and Gene Modules
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ju, Y.; Yue, X.; Min, Z.; Wang, X.; Fang, Y.; Zhang, J. VvNAC17, a novel stress-responsive grapevine (Vitis vinifera L.) NAC transcription factor, increases sensitivity to abscisic acid and enhances salinity, freezing, and drought tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 2020, 146, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Xie, H.; Xiang, N.; Wang, M.; Han, S.; Pan, M.; Guo, X.; Zhang, W. Dynamic changes in anthocyanin accumulation and cellular antioxidant activities in two varieties of grape berries during fruit maturation under different climates. Molecules 2022, 27, 384. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Palomo, E.; Diaz-Maroto, M.C.; Perez-Coello, M.S. Rapid determination of volatile compounds in grapes by HS-SPME coupled with GC-MS. Talanta 2005, 66, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Lopez, R. The actual and potential aroma of winemaking grapes. Biomolecules 2019, 9, 818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perestrelo, R.; Barros, A.S.; Rocha, S.M.; Camara, J.S. Establishment of the varietal profile of Vitis vinifera L. grape varieties from different geographical regions based on HS-SPME/GC-qMS combined with chemometric tools. Microchem. J. 2014, 116, 107–117. [Google Scholar] [CrossRef]
- Pedroza, M.A.; Zalacain, A.; Lara, J.F.; Salinas, M.R. Global grape aroma potential and its individual analysis by SBSE–GC–MS. Food Res. Int. 2010, 43, 1003–1008. [Google Scholar] [CrossRef]
- Cai, J.; Zhu, B.-Q.; Wang, Y.-H.; Lu, L.; Lan, Y.-B.; Reeves, M.J.; Duan, C.-Q. Influence of pre-fermentation cold maceration treatment on aroma compounds of Cabernet Sauvignon wines fermented in different industrial scale fermenters. Food Chem. 2014, 154, 217–229. [Google Scholar] [CrossRef]
- Starkenmann, C.; Le Calve, B.; Niclass, Y.; Cayeux, I.; Beccucci, S.; Troccaz, M. Olfactory perception of cysteine-S-conjugates from fruits and vegetables. J. Agric. Food Chem. 2008, 56, 9575–9580. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Pérez-Álvarez, E.P.; Rubio-Bretón, P.; Garde-Cerdán, T. Changes on grape volatile composition through elicitation with methyl jasmonate, chitosan, and a yeast extract in Tempranillo (Vitis vinifera L.) grapevines. Sci. Hortic. 2019, 244, 257–262. [Google Scholar] [CrossRef]
- Garcia-Esparza, M.J.; Abrisqueta, I.; Escriche, I.; Intrigliolo, D.S.; Alvarez, I.; Lizama, V. Volatile compounds and phenolic composition of skins and seeds of ‘Cabernet Sauvignon’ grapes under different deficit irrigation regimes. Vitis 2018, 57, 83–91. [Google Scholar]
- Xie, S.; Lei, Y.; Wang, Y.; Wang, X.; Ren, R.; Zhang, Z. Influence of continental climates on the volatile profile of Cabernet Sauvignon grapes from five Chinese viticulture regions. Plant Growth Regul. 2019, 87, 83–92. [Google Scholar] [CrossRef]
- Kask, K.; Kaennaste, A.; Talts, E.; Copolovici, L.; Niinemets, U. How specialized volatiles respond to chronic and short-term physiological and shock heat stress in Brassica nigra. Plant Cell Environ. 2016, 39, 2027–2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copolovici, L.; Kaennaste, A.; Pazouki, L.; Niinemets, U. Emissions of green leaf volatiles and terpenoids from Solanum lycopersicum are quantitatively related to the severity of cold and heat shock treatments. J. Plant Physiol. 2012, 169, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, A.; Higaki, T.; Koeduka, T.; Ishigami, K.; Hosokawa, S.; Watanabe, H.; Matsui, K.; Hasezawa, S.; Touhara, K. Transcriptional regulators involved in responses to volatile organic compounds in plants. J. Biol. Chem. 2019, 294, 2256–2266. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zeng, L.; Hou, X.; Liao, Y.; Yang, Z. Low temperature synergistically promotes wounding-induced indole accumulation by INDUCER OF CBF EXPRESSION-mediated alterations of jasmonic acid signaling in Camellia sinensis. J. Exp. Bot. 2020, 71, 2172–2185. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Z.; Kallio, H.; Lu, D.G.; Zhou, C.S.; Ou, S.Y.; Yang, B.R. Acids, sugars, and sugar alcohols in Chinese Hawthorn (Crataegus spp.) fruits. J. Agric. Food Chem. 2010, 58, 1012–1019. [Google Scholar] [CrossRef]
- Xiang, N.; Hu, J.-G.; Yan, S.; Guo, X. Plant hormones and volatiles response to temperature stress in sweet corn (Zea mays L.) seedlings. J. Agric. Food Chem. 2021, 69, 6779–6790. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [Green Version]
- Ali, K.; Maltese, F.; Choi, Y.H.; Verpoorte, R. Metabolic constituents of grapevine and grape-derived products. Phytochem. Rev. 2010, 9, 357–378. [Google Scholar] [CrossRef] [Green Version]
- Munoz-Robredo, P.; Robledo, P.; Manriquez, D.; Molina, R.; Defilippi, B.G. Characterization of sugars and organic acids in commercial varieties of table grapes. Chil. J. Agric. Res. 2011, 71, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Zha, Q.; Xi, X.J.; He, Y.; Jiang, A.L. Bagging affecting sugar and anthocyanin metabolism in the ripening period of grape berries. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 1194–1205. [Google Scholar] [CrossRef] [Green Version]
- Yue, X.; Ren, R.; Ma, X.; Fang, Y.; Zhang, Z.; Ju, Y. Dynamic changes in monoterpene accumulation and biosynthesis during grape ripening in three Vitis vinifera L. cultivars. Food Res. Int. 2020, 137, 109736. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Jin, X.; Feng, M.; Xu, G.; Zhang, P.; Fang, Y.; Xu, T.; Meng, J. Evolution of volatile profile and aroma potential of table grape Hutai-8 during berry ripening. Food Res. Int. 2021, 143, 110330. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Zhang, K.K.; Ji, X.H.; Wang, X.D.; Shi, X.B.; Wang, B.L.; Zheng, X.C.; Liu, F.Z. Effects of different color paper bags on volatile constituents of Kyoho grape berries. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2017, 28, 1274–1280. [Google Scholar]
- Zhao, S.; Wang, X.; Yan, X.; Guo, L.; Mi, X.; Xu, Q.; Zhu, J.; Wu, A.; Liu, L.; Wei, C. Revealing of MicroRNA involved regulatory gene networks on terpenoid biosynthesis in Camellia sinensis in different growing time points. J. Agric. Food Chem. 2018, 66, 12604–12616. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-Y.; Wu, J.-R.; Ngoc Kieu Thi, D.; Lu, C.-A.; Yeh, C.-H.; Wu, S.-J. HSP70-4 and farnesylated AtJ3 constitute a specific HSP70/HSP40-based chaperone machinery essential for prolonged heat stress tolerance in Arabidopsis. J. Plant Physiol. 2021, 261, 153430. [Google Scholar] [CrossRef]
- Giarola, V.; Jung, N.U.; Singh, A.; Satpathy, P.; Bartels, D. Analysis of pcC13-62 promoters predicts a link between cis-element variations and desiccation tolerance in Linderniaceae. J. Exp. Bot. 2018, 69, 3773–3784. [Google Scholar] [CrossRef]
- Huang, T.; Yu, D.; Wang, X. VvWRKY22 transcription factor interacts with VvSnRK1.1/VvSnRK1.2 and regulates sugar accumulation in grape. Biochem. Biophys. Res. Commun. 2021, 554, 193–198. [Google Scholar] [CrossRef]
- Irimia, L.M.; Patriche, C.V.; Bucur, G.M.; Quenol, H.; Cotea, V.V. Spatial distribution of grapes sugar content and its correlations with climate characteristics and climate suitability in the Husi (Romania) wine growing region. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 250–258. [Google Scholar] [CrossRef] [Green Version]
- Min, J.-H.; Ju, H.-W.; Yoon, D.; Lee, K.-H.; Lee, S.; Kim, C.S. Arabidopsis basic helix-loop-helix 34 (bHLH34) is involved in glucose signaling through binding to a GAGA cis-element. Front. Plant Sci. 2017, 8, 2100. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Kang, J.; Xie, Q.; Gong, J.; Shen, H.; Chen, Y.; Chen, G.; Hu, Z. The basic helix-loop-helix transcription factor bHLH95 affects fruit ripening and multiple metabolisms in tomato. J. Exp. Bot. 2020, 71, 6311–6327. [Google Scholar] [CrossRef] [PubMed]
- Matus, J.T.; Aquea, F.; Arce-Johnson, P. Analysis of the grape MYB R2R3 subfamily reveals expanded wine quality-related clades and conserved gene structure organization across Vitis and Arabidopsis genomes. BMC Plant Biol. 2008, 8, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azuma, A.; Yakushiji, H.; Koshita, Y.; Kobayashi, S. Flavonoid biosynthesis-related genes in grape skin are differentially regulated by temperature and light conditions. Planta 2012, 236, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, W.; Min, Z.; Cheng, X.; Fang, Y. Identification and expression analysis of ERECTA family genes in grape (Vitis vinifera L.). Genes Genom. 2019, 41, 723–735. [Google Scholar] [CrossRef]
- Liu, G.-T.; Wang, J.-F.; Cramer, G.; Dai, Z.-W.; Duan, W.; Xu, H.-G.; Wu, B.-H.; Fan, P.-G.; Wang, L.-J.; Li, S.-H. Transcriptomic analysis of grape (Vitis vinifera L.) leaves during and after recovery from heat stress. BMC Plant Biol. 2012, 12, 174. [Google Scholar] [CrossRef] [Green Version]
- Bassolino, L.; Giacomelli, E.; Giovanelli, S.; Pistelli, L.; Cassetti, A.; Damonte, G.; Bisio, A.; Ruffoni, B. Tissue culture and aromatic profile in Salvia dolomitica Codd. Plant Cell Tissue Organ Cult. 2015, 121, 83–95. [Google Scholar] [CrossRef]




| Site | Period | Fructose | Glucose | Malic Acid | Tartaric Acid | Citric Acid |
|---|---|---|---|---|---|---|
| US | S1 | 2.52 ± 0.27 d* | 12.88 ± 3.75 c | 21.58 ± 1.53 a | 18.28 ± 1.68 a | 1.41 ± 0.21 a |
| S2 | 27.68 ± 1.86 c | 37.05 ± 2.87 b | 4.51 ± 0.51 c | 10.49 ± 0.75 bc | ND | |
| S3 | 53.19 ± 3.64 a | 59.36 ± 5.85 a | 2.52 ± 0.22 de | 6.53 ± 0.30 e | ND | |
| S4 | 56.41 ± 6.28 a | 62.36 ± 6.90 a | 1.65 ± 0.22 e | 6.26 ± 0.32 e | ND | |
| S5 | 53.60 ± 2.62 a | 58.49 ± 4.74 a | 2.06 ± 0.31 e | 6.11 ± 0.14 e | ND | |
| TS | S1 | 27.94 ± 1.23 c | 36.86 ± 0.35 b | 6.22 ± 0.49 b | 11.48 ± 1.47 b | 0.51 ± 0.07 b |
| S2 | 39.66 ± 3.43 b | 50.49 ± 4.46 ab | 3.70 ± 0.33 cd | 8.95 ± 0.35 cd | ND | |
| S3 | 49.45 ± 3.47 ab | 51.49 ± 7.80 ab | 2.44 ± 0.44 de | 6.59 ± 0.52 e | ND | |
| S4 | 53.03 ± 2.98 a | 61.93 ± 5.33 a | 1.91 ± 0.15 e | 6.69 ± 0.39 de | ND | |
| S5 | 59.44 ± 7.28 a | 65.06 ± 6.53 a | 2.03 ± 0.09 e | 6.34 ± 0.59 e | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, N.; Xie, H.; Qin, L.; Wang, M.; Guo, X.; Zhang, W. Effect of Climate on Volatile Metabolism in ‘Red Globe’ Grapes (Vitis vinifera L.) during Fruit Development. Foods 2022, 11, 1435. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11101435
Xiang N, Xie H, Qin L, Wang M, Guo X, Zhang W. Effect of Climate on Volatile Metabolism in ‘Red Globe’ Grapes (Vitis vinifera L.) during Fruit Development. Foods. 2022; 11(10):1435. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11101435
Chicago/Turabian StyleXiang, Nan, Hui Xie, Liuwei Qin, Min Wang, Xinbo Guo, and Wen Zhang. 2022. "Effect of Climate on Volatile Metabolism in ‘Red Globe’ Grapes (Vitis vinifera L.) during Fruit Development" Foods 11, no. 10: 1435. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11101435