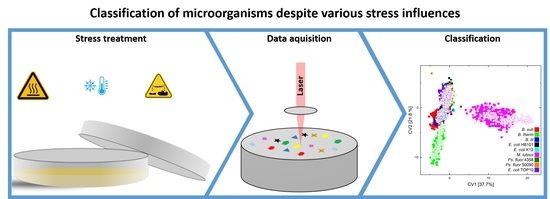
Discrimination of Stressed and Non-Stressed Food-Related Bacteria Using Raman-Microspectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Cultures and Sample Preparation
2.2. Sample Treatment
2.2.1. Lifetime Stress Conditions
2.2.2. Sampling Stress Conditions
2.3. Instrumentation
2.4. Data Handling and Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Candoğan, K.; Altuntas, E.G.; İğci, N. Authentication and quality assessment of meat products by Fourier-Transform infrared (FTIR) spectroscopy. Food Eng. Rev. 2020, 13, 66–91. [Google Scholar] [CrossRef]
- Stenmarck, Å.; Jensen, C.; Quested, T.; Moates, G.; Cseh, B.; Juul, S.; Parry, A.; Politano, A.; Redlingshofer, B.; Scherhaufer, S.; et al. Estimates of European Food Waste Levels; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2016; ISBN 9789188319012. [Google Scholar]
- Buettner, T. World population prospects—A long view. Econ. Stat./Econ. Stat. 2020, 520–521, 9–27. [Google Scholar] [CrossRef]
- European Commission Internal Market, Industry, Entrepreneurship and SMEs—Food and Drink Industry. Available online: https://ec.europa.eu/growth/smes/business-friendly-environment/sme-definition_en (accessed on 11 May 2022).
- European Commission, Directorate-General for Health and Food Safety. Food Waste and Date Marking: Summary, Publications Office. 2015. Available online: https://data.europa.eu/doi/10.2875/25093 (accessed on 28 September 2020).
- World Health Organisation Food Safety. Fact Sheet Number 399. Available online: http://www.who.int/mediacentre/factsheets/fs399/en/ (accessed on 28 September 2020).
- Forbes, H.; Quested, T.; O’Connor, C. Food Waste Index Report 2021; United Nations Environment Programme (2021): Nairobi, Kenya, 2021. [Google Scholar]
- Alvarez-Ordóñez, A.; Mouwen, D.J.M.J.M.; López, M.; Prieto, M. Fourier transform infrared spectroscopy as a tool to characterize molecular composition and stress response in foodborne pathogenic bacteria. J. Microbiol. Methods 2011, 84, 369–378. [Google Scholar] [CrossRef]
- Troy, D.J.; Ojha, K.S.; Kerry, J.P.; Tiwari, B.K. Sustainable and consumer-friendly emerging technologies for application within the meat industry: An overview. Meat Sci. 2016, 120, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Pahlow, S.; Meisel, S.; Cialla-May, D.; Weber, K.; Rösch, P.; Popp, J. Isolation and identification of bacteria by means of Raman spectroscopy. Adv. Drug Deliv. Rev. 2015, 89, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Maquelin, K.; Kirschner, C.; Choo-Smith, L.-P.; van den Braak, N.; Endtz, H.P.; Naumann, D.; Puppels, G. Identification of medically relevant microorganisms by vibrational spectroscopy. J. Microbiol. Methods 2002, 51, 255–271. [Google Scholar] [CrossRef]
- Hameed, S.; Xie, L.; Ying, Y. Conventional and emerging detection techniques for pathogenic bacteria in food science: A review. Trends Food Sci. Technol. 2018, 81, 61–73. [Google Scholar] [CrossRef]
- Ajaykumar, V.J.; Mandal, P.K. Modern concept and detection of spoilage in meat and meat products. In Meat Quality Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 335–349. ISBN 9780128192337. [Google Scholar]
- Hlaing, M.M.; Dunn, M.; Stoddart, P.R.; McArthur, S.L. Raman spectroscopic identification of single bacterial cells at different stages of their lifecycle. Vib. Spectrosc. 2016, 86, 81–89. [Google Scholar] [CrossRef]
- Yamamoto, T.; Taylor, J.N.; Koseki, S.; Koyama, K. Classification of food spoilage bacterial species and their sodium chloride, sodium acetate and glycine tolerance using chemometrics analysis and Raman spectroscopy. J. Microbiol. Methods 2021, 190, 106326. [Google Scholar] [CrossRef]
- Hong, J.-K.; Kim, S.B.; Lyou, E.S.; Lee, T.K. Microbial phenomics linking the phenotype to function: The potential of Raman spectroscopy. J. Microbiol. 2021, 59, 249–258. [Google Scholar] [CrossRef]
- Price, P.B.; Sowers, T. Temperature dependence of metabolic rates for microbial growth, maintenance, and survival. Proc. Natl. Acad. Sci. USA 2004, 101, 4631–4636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boor, K.J. Bacterial stress responses: What doesn’t kill them can make them stronger. PLoS Biol. 2006, 4, e23. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wu, K.; Lee, C. Stress-responsive periplasmic chaperones in bacteria. Front. Mol. Biosci. 2021, 8, 1–14. [Google Scholar] [CrossRef]
- Oliver, J.D. The viable but nonculturable state in bacteria. J. Microbiol. 2005, 43, 93–100. [Google Scholar] [PubMed]
- Lu, X.; Al-Qadiri, H.M.; Lin, M.; Rasco, B.A. Application of mid-infrared and Raman spectroscopy to the study of bacteria. Food Bioprocess Technol. 2011, 4, 919–935. [Google Scholar] [CrossRef]
- Wichmann, C.; Chhallani, M.; Bocklitz, T.; Rösch, P.; Popp, J. Simulation of transportation and storage and their influence on Raman spectra of bacteria. Anal. Chem. 2019, 91, 13688–13694. [Google Scholar] [CrossRef] [PubMed]
- Naumann, D. Infrared Spectroscopy in Microbiology. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2006; pp. 1–29. ISBN 9780470027318. [Google Scholar]
- Argyri, A.A.; Jarvis, R.M.; Wedge, D.; Xu, Y.; Panagou, E.Z.; Goodacre, R.; Nychas, G.J.E.J.E. A comparison of Raman and FT-IR spectroscopy for the prediction of meat spoilage. Food Control 2013, 29, 461–470. [Google Scholar] [CrossRef]
- Münchberg, U.; Kloß, S.; Kusić, D.; Meisel, S.; Heinke, R.; Stöckel, S.; Rösch, P.; Popp, J. IR and Raman spectroscopy for pathogen detection. In Modern Techniques for Pathogen Detection; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 253–294. ISBN 9783527687978. [Google Scholar]
- Wang, K.; Sun, D.-W. Imaging Spectroscopic Technique: Raman Chemical Imaging, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Schulz, H. Spectroscopic Technique: Raman Spectroscopy, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 139–191. [Google Scholar]
- Davis, R.; Mauer, L. Fourier transform infrared (FT-IR) spectroscopy: A rapid tool for detection and analysis of foodborne pathogenic bacteria. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. A. Méndez-Vilas 2010, 2, 1582–1594. [Google Scholar]
- Harrison, J.P.; Berry, D. Vibrational spectroscopy for imaging single microbial cells in complex biological samples. Front. Microbiol. 2017, 8, 675. [Google Scholar] [CrossRef] [Green Version]
- Larkin, P. Infrared and Raman Spectroscopy; Elsevier Inc.: Amsterdam, The Nederlands, 2011; ISBN 9780123869845. [Google Scholar]
- Helm, D.; Labischinski, H.; Naumann, D. Elaboration of a procedure for identification of bacteria using Fourier-Transform IR spectral libraries: A stepwise correlation approach. J. Microbiol. Methods 1991, 14, 127–142. [Google Scholar] [CrossRef]
- Florence, J.M.; Allshouse, C.C.; Glaze, F.W.; Hahner, C.H. Absorption of near-infrared energy by certain glasses. J. Res. Natl. Bur. Stand. 1950, 45, 121. [Google Scholar] [CrossRef]
- Jarvis, R.M.; Goodacre, R. Discrimination of Bacteria Using Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2004, 76, 40–47. [Google Scholar] [CrossRef]
- Skoog, D.A.; Holler, F.J.; Crouch, S.R. Principles of Instrumental Analysis, 6th ed.; Thomson Higher Education: Belmont, CA, USA, 2007; ISBN 9788578110796. [Google Scholar]
- Naumann, D.; Keller, S.; Helm, D.; Schultz, C.; Schrader, B. FT-IR spectroscopy and FT-Raman spectroscopy are powerful analytical tools for the non-invasive characterization of intact microbial cells. J. Mol. Struct. 1995, 347, 399–405. [Google Scholar] [CrossRef]
- Harz, M.; Rösch, P.; Peschke, K.-D.; Ronneberger, O.; Burkhardt, H.; Popp, J. Micro-Raman spectroscopic identification of bacterial cells of the genus Staphylococcus and dependence on their cultivation conditions. Analyst 2005, 130, 1543. [Google Scholar] [CrossRef] [PubMed]
- Sil, S.; Mukherjee, R.; Kumar, N.S.; Aravind, S.; Kingston, J.; Singh, U.K. Detection and classification of bacteria using Raman spectroscopy combined with multivariate analysis. Def. Life Sci. J. 2017, 2, 435. [Google Scholar] [CrossRef] [Green Version]
- Breuch, R.; Klein, D.; Siefke, E.; Hebel, M.; Herbert, U.; Wickleder, C.; Kaul, P. Differentiation of meat-related microorganisms using paper-based surface-enhanced Raman spectroscopy combined with multivariate statistical analysis. Talanta 2020, 219, 1–7. [Google Scholar] [CrossRef]
- Meisel, S.; Stöckel, S.; Rösch, P.; Popp, J. Identification of meat-associated pathogens via Raman microspectroscopy. Food Microbiol. 2014, 38, 36–43. [Google Scholar] [CrossRef]
- Klein, D.; Breuch, R.; von der Mark, S.; Wickleder, C.; Kaul, P. Detection of spoilage associated bacteria using Raman-microspectroscopy combined with multivariate statistical analysis. Talanta 2019, 196, 325–328. [Google Scholar] [CrossRef]
- Lorenz, B.; Wichmann, C.; Stöckel, S.; Rösch, P.; Popp, J. Cultivation-free Raman spectroscopic investigations of bacteria. Trends Microbiol. 2017, 25, 413–424. [Google Scholar] [CrossRef]
- Athamneh, A.I.M.; Alajlouni, R.A.; Wallace, R.S.; Seleem, M.N.; Senger, R.S. Phenotypic profiling of antibiotic response signatures in Escherichia coli using Raman spectroscopy. Antimicrob. Agents Chemother. 2014, 58, 1302–1314. [Google Scholar] [CrossRef] [Green Version]
- Walter, A.; Reinicke, M.; Bocklitz, T.; Schumacher, W.; Rösch, P.; Kothe, E.; Popp, J. Raman spectroscopic detection of physiology changes in plasmid-bearing Escherichia coli with and without antibiotic treatment. Anal. Bioanal. Chem. 2011, 400, 2763–2773. [Google Scholar] [CrossRef] [PubMed]
- Verma, T.; Annappa, H.; Singh, S.; Umapathy, S.; Nandi, D. Profiling antibiotic resistance in Escherichia coli strains displaying differential antibiotic susceptibilities using Raman spectroscopy. J. Biophotonics 2021, 14, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Wang, X.; Wang, X.; Gou, H.; Ren, L.; Wang, T.; Wang, Y.; Ji, Y.; Huang, W.E.; Xu, J. Label-free, rapid and quantitative phenotyping of stress response in E. coli via ramanome. Sci. Rep. 2016, 6, 34359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Timermans, C.; Rubbens, P.; Kerckhof, F.-M.; Buysschaert, B.; Khalenkow, D.; Waegeman, W.; Skirtach, A.G.; Boon, N. Label-free Raman characterization of bacteria calls for standardized procedures. J. Microbiol. Methods 2018, 151, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, C.; Bocklitz, T.; Rösch, P.; Popp, J. Bacterial phenotype dependency from CO2 measured by Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248, 119170. [Google Scholar] [CrossRef] [PubMed]
- Zu, T.N.K.; Athamneh, A.I.M.; Wallace, R.S.; Collakova, E.; Senger, R.S. Near-real-time analysis of the phenotypic responses of Escherichia coli to 1-butanol exposure using Raman spectroscopy. J. Bacteriol. 2014, 196, 3983–3991. [Google Scholar] [CrossRef] [Green Version]
- Zu, T.N.K.; Athamneh, A.I.M.; Senger, R.S. Characterizing the phenotypic responses of Escherichia coli to multiple 4-carbon alcohols with raman spectroscopy. Fermentation 2016, 2, 3. [Google Scholar] [CrossRef]
- Mukherjee, R.; Verma, T.; Nandi, D.; Umapathy, S. Understanding the effects of culture conditions in bacterial growth: A biochemical perspective using Raman microscopy. J. Biophotonics 2020, 13, 1–11. [Google Scholar] [CrossRef]
- Němcová, A.; Gonová, D.; Samek, O.; Sipiczki, M.; Breierová, E.; Márová, I. The use of Raman spectroscopy to monitor metabolic changes in stressed Metschnikowia sp. yeasts. Microorganisms 2021, 9, 277. [Google Scholar] [CrossRef]
- Li, R.; Dhankhar, D.; Chen, J.; Krishnamoorthi, A.; Cesario, T.C.; Rentzepis, P.M. Identification of live and dead bacteria: A Raman spectroscopic study. IEEE Access 2019, 7, 23549–23559. [Google Scholar] [CrossRef]
- Ryu, Y.; Hong, M.; Kim, S.B.; Lee, T.K.; Park, W. Raman spectroscopy reveals alteration of spore compositions under different nutritional conditions in Lysinibacillus boronitolerans YS11. J. Microbiol. 2021, 59, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Tanniche, I.; Collakova, E.; Denbow, C.; Senger, R.S. Characterizing metabolic stress-induced phenotypes of Synechocystis PCC6803 with Raman spectroscopy. Peer J. 2020, 8, e8535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, D.; Breuch, R.; Reinmüller, J.; Engelhard, C.; Kaul, P. Rapid detection and discrimination of food-related bacteria using IR-microspectroscopy in combination with multivariate statistical analysis. Talanta 2021, 232, 122424. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Doksum, K.; Tsui, K.W. Principal Component Analysis (PCA) for high dimensional data. PCA is dead. Long live PCA. Perspect. Big Data Anal. Methodol. Appl. 2014, 622, 1–10. [Google Scholar]
- Fisher, R.A. The use of multiple measurements in taxonomic problems. Ann. Eugen. 1936, 7, 179–188. [Google Scholar] [CrossRef]
- Backhaus, K.; Erichson, B.; Plinke, W.; Weiber, R. Multivariate Analysemethoden. In Multivariate Analysemethoden; Springer: Berlin/Heidelberg, Germany, 2011; pp. 188–247. ISBN 978-3-642-16490-3. [Google Scholar]
- Escoriza, M.F.; Van Briesen, J.M.; Stewart, S.; Maier, J. Raman spectroscopic discrimination of cell response to chemical and physical inactivation. Appl. Spectrosc. 2007, 61, 812–823. [Google Scholar] [CrossRef]
- Mosier-Boss, P. Review on SERS of bacteria. Biosensors 2017, 7, 51. [Google Scholar] [CrossRef] [Green Version]
- Withnall, R.; Chowdhry, B.Z.; Silver, J.; Edwards, H.G.M.; De Oliveira, L.F.C. Raman spectra of carotenoids in natural products. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2003, 59, 2207–2212. [Google Scholar] [CrossRef]
- Netzer, R.; Stafsnes, M.H.; Andreassen, T.; Goksøyr, A.; Bruheim, P.; Brautaset, T. Biosynthetic pathway for γ-cyclic sarcinaxanthin in Micrococcus luteus: Heterologous expression and evidence for diverse and multiple catalytic functions of C 50 carotenoid cyclases. J. Bacteriol. 2010, 192, 5688–5699. [Google Scholar] [CrossRef] [Green Version]
- Schuster, K.C.; Reese, I.; Urlaub, E.; Gapes, J.R.; Lendl, B. Multidimensional information on the chemical composition of single bacterial cells by confocal Raman microspectroscopy. Anal. Chem. 2000, 72, 5529–5534. [Google Scholar] [CrossRef]
- Malyshev, D.; Öberg, R.; Dahlberg, T.; Wiklund, K.; Landström, L.; Andersson, P.O.; Andersson, M. Laser induced degradation of bacterial spores during micro-Raman spectroscopy. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2022, 265, 120381. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhang, P.; Wang, G.; Yu, J.; Setlow, P.; Li, Y.Q. Characterization of bacterial spore germination using phase-contrast and fluorescence microscopy, Raman spectroscopy and optical tweezers. Nat. Protoc. 2011, 6, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Setlow, P.; Li, Y. Characterization of single heat-activated Bacillus spores using laser tweezers Raman spectroscopy. Opt. Express 2009, 17, 16480. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Setlow, P.; Li, Y. Analysis of the Raman spectra of Ca2+-dipicolinic acid alone and in the bacterial spore core in both aqueous and dehydrated environments. Analyst 2012, 137, 3683. [Google Scholar] [CrossRef]
- Maquelin, K.; Choo-Smith, L.-P.; van Vreeswijk, T.; Endtz, H.P.; Smith, B.; Bennett, R.; Bruining, H.A.; Puppels, G.J. Raman spectroscopic method for identification of clinically relevant microorganisms growing on solid culture medium. Anal. Chem. 2000, 72, 12–19. [Google Scholar] [CrossRef]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Moens, L. Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. 2007, 38, 1133–1147. [Google Scholar] [CrossRef]
- Fan, C.; Hu, Z.; Mustapha, A.; Lin, M. Rapid detection of food- and waterborne bacteria using surface-enhanced Raman spectroscopy coupled with silver nanosubstrates. Appl. Microbiol. Biotechnol. 2011, 92, 1053–1061. [Google Scholar] [CrossRef]
- Ryabchykov, O.; Guo, S.; Bocklitz, T. Analyzing Raman spectroscopic data. Phys. Sci. Rev. 2019, 4, 1–10. [Google Scholar] [CrossRef]
- Butler, H.J.; Ashton, L.; Bird, B.; Cinque, G.; Curtis, K.; Dorney, J.; Esmonde-White, K.; Fullwood, N.J.; Gardner, B.; Martin-Hirsch, P.L.; et al. Using Raman spectroscopy to characterize biological materials. Nat. Protoc. 2016, 11, 664–687. [Google Scholar] [CrossRef] [Green Version]
- Hlaing, M.M.; Wood, B.R.; McNaughton, D.; Rood, J.I.; Fox, E.M.; Augustin, M.A. Vibrational spectroscopy combined with transcriptomic analysis for investigation of bacterial responses towards acid stress. Appl. Microbiol. Biotechnol. 2018, 102, 333–343. [Google Scholar] [CrossRef]
- Strola, S.A.; Baritaux, J.-C.; Schultz, E.; Simon, A.C.; Allier, C.; Espagnon, I.; Jary, D.; Dinten, J.-M. Single bacteria identification by Raman spectroscopy. J. Biomed. Opt. 2014, 19, 111610. [Google Scholar] [CrossRef] [PubMed]




| Predicted Class | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Class | B. sub | B. therm | B. tii | E. coli. HB101 | E. coli K12 | M. luteus | Ps. fluor 4358 | Ps. fluor 50090 | E. coli TOP10 |
| B. sub | 199 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| B. therm | 0 | 204 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| B. tii | 0 | 0 | 199 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli HB101 | 0 | 0 | 0 | 138 | 62 | 0 | 0 | 0 | 5 |
| E. coli K12 | 0 | 0 | 0 | 24 | 139 | 0 | 0 | 0 | 42 |
| M. luteus | 0 | 0 | 0 | 0 | 0 | 210 | 0 | 0 | 0 |
| Ps. fluor 4358 | 0 | 0 | 0 | 7 | 0 | 0 | 197 | 6 | 0 |
| Ps. fluor 50090 | 0 | 0 | 0 | 0 | 0 | 0 | 29 | 181 | 0 |
| E. coli TOP10 | 0 | 0 | 0 | 3 | 51 | 0 | 0 | 0 | 151 |
| Error Distribution | B. tii | E. coli HB101 | E. coli K12 | Ps. fluor 4358 | Ps. fluor 50090 | E. coli TOP10 |
|---|---|---|---|---|---|---|
| B. sub—cold sampling | 1 | |||||
| B. therm—25 °C | 1 | |||||
| B. tii—heat dried | 1 | |||||
| E. coli HB101—desiccator | 4 | |||||
| E. coli HB101—cold sampling | 1 | 3 | ||||
| E. coli HB101—HCl | 22 | 1 | ||||
| E. coli HB101—heat dried | 1 | |||||
| E. coli HB101—2-propanol | 10 | 1 | ||||
| E. coli HB101—NaOH | 18 | |||||
| E. coli HB101—regular | 6 | |||||
| E. coli K12—desiccator | 5 | |||||
| E. coli K12—HCl | 3 | 9 | ||||
| E. coli K12—heat dried | 1 | 20 | ||||
| E. coli K12—2-propanol | 4 | 1 | ||||
| E. coli K12—NaOH | 16 | 7 | ||||
| Ps. fluor 4358—desiccator | 7 | 6 | ||||
| Ps. fluor 50090—25 °C | 3 | |||||
| Ps. fluor 50090—HCl | 22 | |||||
| Ps. fluor 50090—heat dried | 4 | |||||
| E. coli TOP10—25 °C | 2 | 15 | ||||
| E. coli TOP10—desiccator | 12 | |||||
| E. coli TOP10—heat dried | 1 | |||||
| E. coli TOP10—2-propanol | 1 | |||||
| E. coli TOP10—regular | 1 | 22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, D.; Breuch, R.; Reinmüller, J.; Engelhard, C.; Kaul, P. Discrimination of Stressed and Non-Stressed Food-Related Bacteria Using Raman-Microspectroscopy. Foods 2022, 11, 1506. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11101506
Klein D, Breuch R, Reinmüller J, Engelhard C, Kaul P. Discrimination of Stressed and Non-Stressed Food-Related Bacteria Using Raman-Microspectroscopy. Foods. 2022; 11(10):1506. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11101506
Chicago/Turabian StyleKlein, Daniel, René Breuch, Jessica Reinmüller, Carsten Engelhard, and Peter Kaul. 2022. "Discrimination of Stressed and Non-Stressed Food-Related Bacteria Using Raman-Microspectroscopy" Foods 11, no. 10: 1506. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11101506