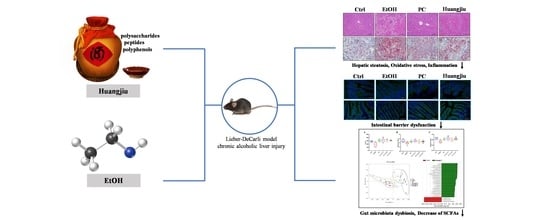
Non-Alcoholic Components in Huangjiu as Potential Factors Regulating the Intestinal Barrier and Gut Microbiota in Mouse Model of Alcoholic Liver Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of the Main Components in Three Types of Huangjiu
2.2. Animal and Experimental Design
2.3. Histopathological and Immunofluorescence Assessment
2.4. Biochemical Analysis
2.5. Estimation of GSH Levels, Antioxidant Enzyme Activities and Lipid Peroxidation
2.6. Hepatic LPS Content Determination
2.7. Pro-Inflammatory Cytokine Level Measurement
2.8. Quantitative RT-PCR Analysis
2.9. Gut Microbiota Assessment
2.10. Quantitative Analysis of SCFAs
2.11. Statistical Analysis
3. Results
3.1. Huangjiu Feeding Increased Body Weight, Lowered the Liver Index, Suppressed Hepatic Steatosis, and Mitigated Intestinal Damage Relative to EtOH Feeding
3.2. Huangjiu Interventions Improved the Hepatic Function Indexes of Mice Relative to EtOH Intervention
3.3. The Antioxidant Defense System of Huangjiu-Treated Mice Was More Complete Than That of EtOH-Treated Mice
3.4. Huangjiu Feeding Resulted in a Lower Level of Hepatic Inflammatory Factors in Mice Than EtOH Feeding
3.5. Huangjiu Interventions Improved the Integrity of Intestinal Barrier in Mice Relative to EtOH Intervention
3.6. Huangjiu and EtOH Treatments Resulted in Divergent Intestinal Community Structures and Gut Microbiota Profiles
3.7. Huangjiu and EtOH Intake Resulted in Distinct SCFA Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, Q.; Wang, X.; Chen, H.; Zhao, C.; Gong, X.; Zhou, X. Structural characterization, modification and hepatoprotective effects of polysaccharide from Mori Fructus. Int. J. Biol. Macromol. 2020, 153, 357–363. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Alcohol and Health 2018; Poznyak, V., Rekve, D., Eds.; World Health Organization: Geneva, Switzerland, 2018; p. 450. ISBN 978-92-4-156563-9. [Google Scholar]
- Gao, B.; Bataller, R. Alcoholic liver disease: Pathogenesis and new therapeutic targets. Gastroenterology 2011, 141, 1572–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Li, H.; Lai, Q.; Yang, Q.; Dong, Y.; Liu, X.; Wang, W.; Zhang, J.; Jia, L. Antioxidant and hepatoprotective activities of modified polysaccharides from Coprinus comatus in mice with alcohol-induced liver injury. Int. J. Biol. Macromol. 2019, 127, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-Y.; Choi, H.-D.; Eom, H.; Choi, I. Enzymatic modification enhances the protective activity of citrus flavonoids against alcohol-induced liver disease. Food Chem. 2013, 139, 231–240. [Google Scholar] [CrossRef]
- Szabo, G. Gut-liver axis in alcoholic liver disease. Gastroenterology 2015, 148, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Saeedi, B.J.; Liu, K.H.; Owens, J.A.; Hunter-Chang, S.; Camacho, M.C.; Eboka, R.U.; Chandrasekharan, B.; Baker, N.F.; Darby, T.M.; Robinson, B.S.; et al. Gut-resident Lactobacilli activate hepatic Nrf2 and protect against oxidative liver injury. Cell Metab. 2020, 31, 956–968. [Google Scholar] [CrossRef]
- Bull-Otterson, L.; Feng, W.; Kirpich, I.; Wang, Y.; Qin, X.; Liu, Y.; Gobejishvili, L.; Joshi-Barve, S.; Ayvaz, T.; Petrosino, J.; et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS ONE 2013, 8, e53028. [Google Scholar] [CrossRef]
- Maccioni, L.; Gao, B.; Leclercq, S.; Pirlot, B.; Horsmans, Y.; De Timary, P.; Leclercq, I.; Fouts, D.; Schnabl, B.; Stärkel, P. Intestinal permeability, microbial translocation, changes in duodenal and fecal microbiota, and their associations with alcoholic liver disease progression in humans. Gut Microbes 2020, 12, 1782157. [Google Scholar] [CrossRef]
- Maldonado, R.F.; Sá-Correia, I.; Valvano, M.A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. Fems Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef]
- Gao, B. Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease. J. Gastroenterol. Hepatol. 2012, 27, 89–93. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.-J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, J.; Zhao, L.; Guan, J.; Liu, F.; Huo, G.; Li, B. Lactobacillus plantarum KLDS1.0344 and Lactobacillus acidophilus KLDS1.0901 mixture prevents chronic alcoholic liver injury in mice by protecting the intestinal barrier and regulating gut microbiota and liver-related pathways. J. Agric. Food Chem. 2020, 69, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Wang, F.; Wong, N.-K.; He, J.; Zhang, R.; Sun, R.; Xu, Y.; Liu, Y.; Li, W.; Koike, K.; et al. Global liver disease burdens and research trends: Analysis from a Chinese perspective. J. Hepatol. 2019, 71, 212–221. [Google Scholar] [CrossRef] [Green Version]
- Gémes, K.; Forsell, Y.; Janszky, I.; László, K.D.; Lundin, A.; Ponce De Leon, A.; Mukamal, K.J.; Möller, J. Moderate alcohol consumption and depression-a longitudinal population-based study in Sweden. Acta Psychiatr. Scand. 2019, 139, 526–535. [Google Scholar] [CrossRef]
- Giacosa, A.; Adam-Blondon, A.F.; Baer-Sinnott, S.; Barale, R.; Bavaresco, L.; Di Gaspero, G.; Dugo, L.; Ellison, R.C.; Gerbi, V.; Gifford, D.; et al. Alcohol and wine in relation to cancer and other diseases. Eur. J. Cancer Prev. 2012, 21, 103–108. [Google Scholar] [CrossRef]
- Millwood, I.Y.; Walters, R.G.; Mei, X.W.; Guo, Y.; Yang, L.; Bian, Z.; Bennett, D.A.; Chen, Y.; Dong, C.; Hu, R.; et al. Conventional and genetic evidence on alcohol and vascular disease aetiology: A prospective study of 500,000 men and women in China. Lancet 2019, 393, 1831–1842. [Google Scholar] [CrossRef] [Green Version]
- De Curtis, A.; Murzilli, S.; Di Castelnuovo, A.; Rotilio, D.; Donati, M.B.; De Gaetano, G.; Iacoviello, L. Alcohol-free red wine prevents arterial thrombosis in dietary-induced hypercholesterolemic rats: Experimental support for the ‘French paradox’. J. Thromb. Haemost. 2005, 3, 346–350. [Google Scholar] [CrossRef]
- Duan, J.; Zhan, J.-C.; Wang, G.-Z.; Zhao, X.-C.; Huang, W.-D.; Zhou, G.-B. The red wine component ellagic acid induces autophagy and exhibits anti-lung cancer activity in vitro and in vivo. J. Cell. Mol. Med. 2019, 23, 143–154. [Google Scholar] [CrossRef]
- Mazué, F.; Delmas, D.; Murillo, G.; Saleiro, D.; Limagne, E.; Latruffe, N. Differential protective effects of red wine polyphenol extracts (RWEs) on colon carcinogenesis. Food Funct. 2014, 5, 663–670. [Google Scholar] [CrossRef]
- Sánchez-Muniz, F.J.; Macho-González, A.; Garcimartín, A.; Santos-López, J.A.; Benedí, J.; Bastida, S.; González-Muñoz, M.J. The nutritional components of beer and Its relationship with neurodegeneration and alzheimer’s disease. Nutrients 2019, 11, 1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lordan, R.; O’Keeffe, E.; Tsoupras, A.; Zabetakis, I. Total, neutral, and polar lipids of brewing ingredients, by-products and beer: Evaluation of antithrombotic activities. Foods 2019, 8, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izu, H.; Hizume, K.; Goto, K.; Hirotsune, M. Hepatoprotective effects of a concentrate and components of sake against galactosamine (GalN)-induced liver injury in mice. Biosci. Biotechnol. Biochem. 2007, 71, 951–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, M.; Bu, T.; Zheng, J.; Liu, L.; Yu, S.; Li, S.; Wu, J. Peptides in brewed wines: Formation, structure, and function. J. Agric. Food Chem. 2021, 69, 2647–2657. [Google Scholar] [CrossRef] [PubMed]
- Han, F.L.; Xu, Y. Identification of low molecular weight peptides in Chinese rice wine (Huang Jiu) by UPLC-ESI-MS/MS. J. Inst. Brew. 2011, 117, 238–250. [Google Scholar] [CrossRef]
- Jin, Z.; Cai, G.; Wu, C.; Hu, Z.; Xu, X.; Xie, G.; Wu, D.; Lu, J. Profiling the key metabolites produced during the modern brewing process of Chinese rice wine. Food Res. Int. 2021, 139, 109955. [Google Scholar] [CrossRef]
- Shen, C.; Mao, J.; Chen, Y.; Meng, X.; Ji, Z. Extraction optimization of polysaccharides from Chinese rice wine from the Shaoxing region and evaluation of its immunity activities. J. Sci. Food Agric. 2015, 95, 1991–1996. [Google Scholar] [CrossRef]
- Xu, Q.; Zhou, Z.; Mao, J.; Yue, C.; Li, Z. Application of SPE-HPLC for determination of phenolic compounds in Chinese rice wines. J. Food Sci. Biotechnol. 2018, 37, 1021–1027. [Google Scholar]
- Shen, C.; Mao, J.; Chen, Y.; Meng, X.; Aisikaer, A. Effect of polysaccharides from Chinese rice wine on immunity-related cytokines in immunodeficient mice. Food Sci. 2015, 36, 158–162. [Google Scholar] [CrossRef]
- Dai, J.; Chen, S.; Xie, G.; Liu, J.; Shuai, G.; Gu, X.; Zhao, G. Isolation and sequence analysis of angiotensin converting enzyme inhibitory peptides in Chinese rice wine. J. Instrum. Anal. 2006, 25, 74–77. [Google Scholar]
- Shi, Y.; Feng, R.; Mao, J.; Liu, S.; Zhou, Z.; Ji, Z.; Chen, S.; Mao, J. Structural characterization of peptides from huangjiu and their regulation of hepatic steatosis and gut microbiota dysbiosis in hyperlipidemia mice. Front. Pharmacol. 2021, 12, 689092. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Zhai, X.; Jiang, C.; Ji, Z.; Guo, Y.; Chi, J.; Guo, H. Exploring the active ingredients in Chinese yellow wine which could inhibit the progress of atherosclerosis in LDLR knockout mice. J. Am. Coll. Cardiol. 2015, 66, C6–C7. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.-W.; Jiang, Y.; Zhang, D.-Y.; Wang, M.; Chen, W.-S.; Su, H.; Wang, Y.-T.; Wan, J.-B. Protective effects of Penthorum chinense Pursh against chronic ethanol-induced liver injury in mice. J. Ethnopharmacol. 2015, 161, 92–98. [Google Scholar] [CrossRef]
- Chen, L.-C.; Zhang, S.-Y.; Zi, Y.; Zhao, H.-M.; Wang, H.-Y.; Zhang, Y. Functional coix seed protein hydrolysates as a novel agent with potential hepatoprotective effect. Food Funct. 2020, 11, 9495–9502. [Google Scholar] [CrossRef]
- Liu, N.; Yang, M.; Huang, W.; Wang, Y.; Yang, M.; Wang, Y.; Zhao, Z. Composition, antioxidant activities and hepatoprotective effects of the water extract of Ziziphus jujuba cv. Jinsixiaozao. RSC Adv. 2017, 7, 6511–6522. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Pan, T.-M. Protective effect of monascus-fermented red mold rice against alcoholic liver disease by attenuating oxidative stress and inflammatory response. J. Agric. Food Chem. 2011, 59, 9950–9957. [Google Scholar] [CrossRef]
- Kiyono, T.; Hirooka, K.; Yamamoto, Y.; Kuniishi, S.; Ohtsuka, M.; Kimura, S.; Park, E.Y.; Nakamura, Y.; Sato, K. Identification of pyroglutamyl peptides in Japanese rice wine (Sake): Presence of hepatoprotective PyroGlu-Leu. J. Agric. Food Chem. 2013, 61, 11660–11667. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, B.; Li, S.; Fang, B.; Duan, W.; Zhang, J.; Song, J.; Wang, M. Vinegar extract ameliorates alcohol-induced liver damage associated with the modulation of gut microbiota in mice. Food Funct. 2020, 11, 2898–2909. [Google Scholar] [CrossRef]
- Xiang, J.; Zhu, W.; Li, Z.; Ling, S. Effect of juice and fermented vinegar from Hovenia dulcis peduncles on chronically alcohol-induced liver damage in mice. Food Funct. 2012, 3, 628–634. [Google Scholar] [CrossRef]
- Gong, J.; Huang, J.; Xiao, G.; You, Y.; Yuan, H.; Chen, F.; Liu, S.; Mao, J.; Li, B. Determination of γ -aminobutyric acid in Chinese rice wines and its evolution during fermentation. J. Inst. Brew. 2017, 123, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Liu, L.; Zhou, C.; Pan, S.; Zhai, X.; Jiang, C.; Guo, Y.; Ji, Z.; Chi, J.; Peng, F.; et al. Polyphenols and polypeptides in Chinese rice wine inhibit homocysteine-induced proliferation and migration of vascular smooth muscle cells. J. Cardiovasc. Pharmacol. 2016, 67, 482–490. [Google Scholar] [CrossRef]
- Louvet, A.; Mathurin, P. Alcoholic liver disease: Mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Feng, X.; Zhang, J.; Wei, Y.; Zhao, X. Preventive effect of Anji white tea flavonoids on alcohol-induced gastric injury through their antioxidant effects in Kunming mice. Biomolecules 2019, 9, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventura-Cots, M.; Rachakonda, V.; Bataller, R. Alcoholic Liver Disease, Management of. In Encyclopedia of Gastroenterology, 2nd ed.; Kuipers, E.J., Ed.; Academic Press: Oxford, UK, 2020; pp. 56–63. [Google Scholar]
- Rao, R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology 2009, 50, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Schnabl, B.; Brenner, D.A. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014, 146, 1513–1524. [Google Scholar] [CrossRef] [Green Version]
- Steed, E.; Balda, M.S.; Matter, K. Dynamics and functions of tight junctions. Trends Cell Biol. 2010, 20, 142–149. [Google Scholar] [CrossRef]
- Atkinson, K.J.; Rao, R.K. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am. J. Physiol.-Gastroint. Liver Physiol. 2001, 280, G1280–G1288. [Google Scholar] [CrossRef]
- Sheth, P.; Seth, A.; Atkinson, K.J.; Gheyi, T.; Kale, G.; Giorgianni, F.; Desiderio, D.M.; Li, C.; Naren, A.; Rao, R. Acetaldehyde dissociates the PTP1B–E-cadherin–β-catenin complex in Caco-2 cell monolayers by a phosphorylation-dependent mechanism. Biochem. J. 2007, 402, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Yan, A.W.; Fouts, D.E.; Brandl, J.; Stärkel, P.; Torralba, M.; Schott, E.; Tsukamoto, H.; Nelson, K.E.; Brenner, D.A.; Schnabl, B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology 2010, 53, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Zhang, R.; Zhou, Q.; Liu, L.; Huang, F.; Deng, Y.; Ma, Y.; Wei, Z.; Tang, X.; Zhang, M. Lychee (Litchi chinensis Sonn.) pulp phenolic extract provides protection against alcoholic liver injury in mice by alleviating intestinal microbiota dysbiosis, intestinal barrier dysfunction, and liver inflammation. J. Agric. Food Chem. 2017, 65, 9675–9684. [Google Scholar] [CrossRef]
- Fang, C.; Du, H.; Zheng, X.; Zhao, A.; Jia, W.; Xu, Y. Solid-state fermented Chinese alcoholic beverage (Baijiu) and ethanol resulted in distinct metabolic and microbiome responses. FASEB J. 2019, 33, 7274–7288. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; He, F.; Zhang, Y.; Xue, J.; Li, K.; Zhang, X.; Zhu, L.; Wang, Z.; Wang, H.; Yang, S. Inulin ameliorates alcoholic liver disease via suppressing LPS-TLR4-Mψ axis and modulating gut microbiota in mice. Alcohol. Clin. Exp. Res. 2018, 43, 411–424. [Google Scholar] [CrossRef]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Chen, S.; Luo, P.; Li, P.; Du, B. Dietary supplementation of Bacillus sp. DU106 activates innate immunity and regulates intestinal microbiota in mice. J. Funct. Food. 2020, 75, 104247. [Google Scholar] [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [Green Version]
- Chemaly, R.F.; Dantes, R.; Shah, D.P.; Shah, P.K.; Pascoe, N.; Ariza-Heredia, E.; Perego, C.; Nguyen, D.B.; Nguyen, K.; Modarai, F.; et al. Cluster and sporadic cases of Herbaspirillum species infections in patients with cancer. Clin. Infect. Dis. 2014, 60, 48–54. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, S.; Zhong, R.; Wan, F.; Chen, L.; Liu, L.; Yi, B.; Zhang, H. Olive fruit extracts supplement improve antioxidant capacity via altering colonic microbiota composition in mice. Front. Nutr. 2021, 8, 645099. [Google Scholar] [CrossRef] [PubMed]
- Dolara, P.; Luceri, C.; Filippo, C.D.; Femia, A.P.; Giovannelli, L.; Caderni, G.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Cresci, A. Red wine polyphenols influence carcinogenesis, intestinal microflora, oxidative damage and gene expression profiles of colonic mucosa in F344 rats. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2005, 591, 237–246. [Google Scholar] [CrossRef]
- Ji, M.; Fang, C.; Jia, W.; Du, H.; Xu, Y. Regulatory effect of volatile compounds in fermented alcoholic beverages on gut microbiota and serum metabolism in mouse model. Food Funct. 2021, 12, 5576–5590. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Zhong, W.; Zheng, X.; Li, Q.; Qiu, Y.; Li, H.; Chen, H.; Zhou, Z.; Jia, W. Chronic ethanol consumption alters mammalian gastrointestinal content metabolites. J. Proteome Res. 2013, 12, 3297–3306. [Google Scholar] [CrossRef] [Green Version]
- Weitkunat, K.; Schumann, S.; Nickel, D.; Kappo, K.A.; Petzke, K.J.; Kipp, A.P.; Blaut, M.; Klaus, S. Importance of propionate for the repression of hepatic lipogenesis and improvement of insulin sensitivity in high-fat diet-induced obesity. Mol. Nutr. Food Res. 2016, 60, 2611–2621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.-Y.; Lannoy, V.; Decobecq, M.-E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.-B.; Wang, P.-Y.; Wang, X.; Wan, Y.-L.; Liu, Y.-C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Qian, J.; Wang, Q.; Wang, F.; Ma, Z.; Qiao, Y. Butyrate protects rat liver against total hepatic ischemia reperfusion injury with bowel congestion. PLoS ONE 2014, 9, e106184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, Y.; Qian, J.; Wang, F.; Ma, Z.; Wang, Q. Butyrate protects liver against ischemia reperfusion injury by inhibiting nuclear factor kappa B activation in Kupffer cells. J. Surg. Res. 2014, 187, 653–659. [Google Scholar] [CrossRef]
- Vernia, P.; Fracasso, P.; Casale, V.; Villotti, G.; Marcheggiano, A.; Stigliano, V.; Pinnaro, P.; Bagnardi, V.; Caprilli, R. Topical butyrate for acute radiation proctitis: Randomised, crossover trial. Lancet 2000, 356, 1232–1235. [Google Scholar] [CrossRef]
- Vernia, P.; Annese, V.; Bresci, G.; d’ Albasio, G.; D’Inca, R.; Giaccari, S.; Ingrosso, M.; Mansi, C.; Riegler, G.; Valpiani, D.; et al. Topical butyrate improves efficacy of 5-ASA in refractory distal ulcerative colitis: Results of a multicentre trial. Eur. J. Clin. Investig. 2003, 33, 244–248. [Google Scholar] [CrossRef]
- Fang, W.; Xue, H.; Chen, X.; Chen, K.; Ling, W. Supplementation with sodium butyrate modulates the composition of the gut microbiota and ameliorates high-fat diet-induced obesity in mice. J. Nutr. 2019, 149, 747–754. [Google Scholar] [CrossRef]
- Cresci, G.A.; Bush, K.; Nagy, L.E. Tributyrin supplementation protects mice from acute ethanol-induced gut injury. Alcohol. Clin. Exp. Res. 2014, 38, 1489–1501. [Google Scholar] [CrossRef]
- Kim, S.; Shin, Y.-C.; Kim, T.-Y.; Kim, Y.; Lee, Y.-S.; Lee, S.-H.; Kim, M.-N.; Eunju, O.; Kim, K.S.; Kweon, M.-N. Mucin degrader Akkermansia muciniphila accelerates intestinal stem cell-mediated epithelial development. Gut Microbes 2021, 13, e1892441. [Google Scholar] [CrossRef]
- Bourriaud, C.; Robins, R.J.; Martin, L.; Kozlowski, F.; Tenailleau, E.; Cherbut, C.; Michel, C. Lactate is mainly fermented to butyrate by human intestinal microfloras but inter-individual variation is evident. J. Appl. Microbiol. 2005, 99, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Sangwan, N.; Stefka, A.T.; Nocerino, R.; Paparo, L.; Aitoro, R.; Calignano, A.; Khan, A.A.; Gilbert, J.A.; Nagler, C.R. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. 2015, 10, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2013, 63, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Marteau, P. Butyrate-producing bacteria as pharmabiotics for inflammatory bowel disease. Gut 2013, 62, 1673. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]










| Assays | Huangjiu A | Huangjiu B | Huangjiu C |
|---|---|---|---|
| Ethanol (% v/v) | 14.24 ± 0.01 b | 13.62 ± 0.07 a | 14.46 ± 0.12 c |
| Total Protein (g/L) | 11.55 ± 0.34 b | 9.71 ± 0.14 a | 12.16 ± 0.15 c |
| Total Phenolics (mg of GAE/L) | 622.35 ± 3.87 b | 572.73 ± 5.76 a | 695.45 ± 3.94 c |
| Free Amino Acids (mg/L) | 2834.74 ± 25.35 a | 3101.98 ± 9.75 b | 3174.10 ± 20.40 c |
| Total Sugars (g/L) | 39.66 ± 0.71 b | 33.95 ± 1.06 a | 39.48 ± 0.71 b |
| Oligosaccharides (g/L) | 3.63 ± 0.08 a | 4.74 ± 0.16 b | 11.83 ± 0.29 c |
| Polysaccharides (g/L) | 9.07 ± 0.26 c | 2.15 ± 0.04 a | 3.85 ± 0.10 b |
| Peptides < 3 kDa (g/L) | 2.22 ± 0.07 a | 2.79 ± 0.05 b | 5.72 ± 0.13 c |
| Gene | Primer Sequences (5′-3′) | Length |
|---|---|---|
| mus GAPDH | F 5′-CCTCGTCCCGTAGACAAAATG-3′ R 5′-TGAGGTCAATGAAGGGGTCGT-3′ | 133 bp |
| mus ZO-1 | F 5′-GGGAAAACCCGAAACTGATG-3′ R 5′-GCTGTACTGTGAGGGCAACG-3′ | 103 bp |
| mus Occludin | F 5′-TCACTTTTCCTGCGGTGACT-3′ R 5′-GGGAACGTGGCCGATATAATG-3′ | 138 bp |
| mus Reg3β | F 5′-GCGCTGAGGCTTCATTCTTGT-3′ R 5′-TGTTACTCCATTCCCATCCACC-3′ | 129 bp |
| mus Reg3γ | F 5′-GTGCCTATGGCTCCTATTGCTA-3′ R 5′-ACCTCTGTTGGGTTCATAGCC-3′ | 221 bp |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhou, Z.; Liu, Y.; Xu, X.; Xu, Y.; Zhou, W.; Chen, S.; Mao, J. Non-Alcoholic Components in Huangjiu as Potential Factors Regulating the Intestinal Barrier and Gut Microbiota in Mouse Model of Alcoholic Liver Injury. Foods 2022, 11, 1537. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11111537
Yang Y, Zhou Z, Liu Y, Xu X, Xu Y, Zhou W, Chen S, Mao J. Non-Alcoholic Components in Huangjiu as Potential Factors Regulating the Intestinal Barrier and Gut Microbiota in Mouse Model of Alcoholic Liver Injury. Foods. 2022; 11(11):1537. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11111537
Chicago/Turabian StyleYang, Yi, Zhilei Zhou, Yufei Liu, Xibiao Xu, Yuezheng Xu, Weibiao Zhou, Shuguang Chen, and Jian Mao. 2022. "Non-Alcoholic Components in Huangjiu as Potential Factors Regulating the Intestinal Barrier and Gut Microbiota in Mouse Model of Alcoholic Liver Injury" Foods 11, no. 11: 1537. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11111537