The Impact of Freeze-Drying Conditions on the Physico-Chemical Properties and Bioactive Compounds of a Freeze-Dried Orange Puree
Abstract
:1. Introduction
2. Materials and Methods
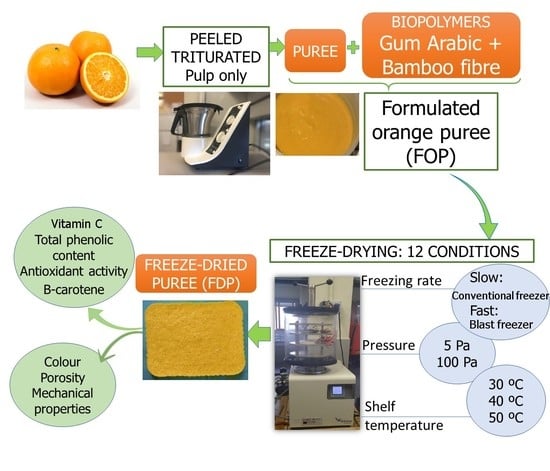
2.1. Raw Materials
2.2. Freeze-Drying Processing
2.3. Water Content
2.4. Mechanical Properties
2.5. Colour Measurements
2.6. Porosity
2.7. Total Polyphenolic Compounds
2.8. Antioxidant Activity
2.9. Vitamin C
2.10. β-Carotene
2.11. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization
3.1.1. Colour
3.1.2. Structure
3.2. Bioactive Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poulose, S.M.; Harris, E.D.; Patil, B.S. Citrus Limonoids Induce Apoptosis in Human Neuroblastoma Cells and Have Radical Scavenging Activity. J. Nutr. 2005, 135, 870–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kader, A.A. Flavor Quality of Fruits and Vegetables. J. Sci. Food Agric. 2008, 88, 1863–1868. [Google Scholar] [CrossRef]
- De Ancos, B.; Gónzalez, E.M.; Cano, M.P. Ellagic Acid, Vitamin C, and Total Phenolic Contents and Radical Scavenging Capacity Affected by Freezing and Frozen Storage in Raspberry Fruit. J. Agric. Food Chem. 2000, 48, 4565–4570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aschoff, J.K.; Kaufmann, S.; Kalkan, O.; Neidhart, S.; Carle, R.; Schweiggert, R.M. In Vitro Bioaccessibility of Carotenoids, Flavonoids, and Vitamin C from Differently Processed Oranges and Orange Juices [Citrus sinensis (L.) Osbeck]. J. Agric. Food Chem. 2015, 63, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Gökmen, V.; Kahraman, N.; Demir, N.; Acar, J. Enzymatically Validated Liquid Chromatographic Method for the Determination of Ascorbic and Dehydroascorbic Acids in Fruit and Vegetables. J. Chromatogr. A 2000, 881, 309–316. [Google Scholar] [CrossRef]
- Peterson, J.; Dwyer, J.; Beecher, G.; Bhagwat, S.A.; Gebhhardt, S.E.; Haytowitz, D.B.; Holden, J.M. Flavanones in Oranges, Tangerines (Mandarins), Tangors, and Tangelos: A Compilation and Review of the Data from the Analytical Literature. J. Food Compos. Anal. 2006, 19, 66–73. [Google Scholar] [CrossRef]
- Ramful, D.; Tarnus, E.; Aruoma, O.I.; Bourdon, E.; Bahorun, T. Polyophenol Composition, Vitamin C Content and Antioxidant Capacity of Mauritian Citrus Fruit Pulps. Food Res. Int. 2011, 44, 2088–2099. [Google Scholar] [CrossRef]
- Goulas, V.; Manganaris, G.A. Exploring the Phytochemical Content and the Antioxidant Potential of Citrus Fruits Grown in Cyprus. Food Chem. 2012, 131, 39–47. [Google Scholar] [CrossRef]
- Moreiras, O.; Carbajal, A.; Cabrera, L.; Cuadrado, C. Tablas de Composición de los Alimentos; Pirámide Editions: Madrid, Spain, 2018; pp. 52–53. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; The National Academies Press: Washington, DC, USA, 2000; p. 507.
- Fazaeli, M.; Emam-Djomeh, Z.; Kalbasi-Ashtari, A.; Omid, M. Effect of Process Conditions and Carrier Concentration for Improving Drying Yield and other Quality Attributes of Spray Dried Black Mulberry (Morus nigra) Juice. Int. J. Food Eng. 2012, 8, 1–20. [Google Scholar] [CrossRef]
- Barbosa-Canovas, G.; Ortega-Rivas, E.; Juliano, P.; Yan, H. Food Powders: Physical Properties, Processing and Functionality; Kluwer Academic/Plenum Publisher: New York, NY, USA, 2005; pp. 271–304. [Google Scholar]
- Karam, M.C.; Petit, J.; Zimmer, D.; Baudelaire, E. Effects of Drying and Grinding in Production of Fruit and Vegetable Powders. A Review. J. Food Eng. 2016, 188, 32–49. [Google Scholar] [CrossRef]
- Roos, Y.H. Phase Transitions in Food; Academic Press: San Diego, CA, USA, 1995; pp. 19–47. [Google Scholar]
- Silva, M.A.; Sobral, P.J.A.; Kieckbusch, T.G. State Diagrams of Freeze-Dried Camu-Camu (Myrciaria dubia (HBK) Mc Vaugh) Pulp with and without Maltodextrin Addition. J. Food Eng. 2006, 77, 426–432. [Google Scholar] [CrossRef]
- Conceição, M.C.; Fernandes, T.N.; de Resende, J.V. Stability and Microstructure of Freeze-Dried Guava Pulp (Psidium guajava, L.) with Added Sucrose and Pectin. J. Food Sci. Technol. 2016, 53, 2654–2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telis, V.R.N.; Martínez-Navarrete, N. Biopolymers Used as Drying Aids in Spray- Drying and Freeze-Drying of Fruit Juices and Pulps. In Biopolymer Engineering in Food Processing; Telis, V.N.N., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 279–325. [Google Scholar]
- Martínez-Navarrete, N.; Salvador, A.; Oliva, C.; Camacho, M.M. Influence of Biopolymers and Freeze-Drying Shelf Temperature on the Quality of a Mandarin Snack. LWT 2019, 99, 57–61. [Google Scholar] [CrossRef]
- Genin, N.; Rene, F. Influence of Freezing Rate and the Ripeness State of Fresh Courgette on the Quality of Freeze-Dried Products and Freeze-Drying Time. J. Food Eng. 1996, 29, 201–209. [Google Scholar] [CrossRef]
- Karel, M.; Flink, J.M. Influence of Frozen State Reactions on Freeze-Dried Foods. J. Agric. Food Chem. 1973, 21, 16–21. [Google Scholar] [CrossRef]
- Ceballos, A.M.; Giraldo, G.I.; Orrego, C.E. Effect of Freezing Rate on Quality Parameters of Freeze-Dried Soursop Fruit Pulp. J. Food Eng. 2012, 111, 360–365. [Google Scholar] [CrossRef]
- Hammami, C.; René, F. Determination of Freeze-Drying Process Variables for Strawberries. J. Food Eng. 1997, 32, 133–154. [Google Scholar] [CrossRef]
- Lombraña, J.I. The Influence of Pressure and Temperature on Freeze-Drying in an Adsorbent Medium and Establishment of Drying Strategies. Food Res. Int. 1997, 30, 213–222. [Google Scholar] [CrossRef]
- Ratti, C. Hot Air and Freeze-Drying of High-Value Foods: A Review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Egas-Astudillo, L.A.; Silva, A.; Uscanga, M.; Martínez-Navarrete, N.; Camacho, M.M. Impact of Shelf Temperature on Freeze-Drying Process and Porosity Development. In Proceedings of the 21st International Drying Symposium (IDS’ 2018), Valencia, Spain, 11–14 September 2018; pp. 843–850. [Google Scholar]
- Agudelo, C.; Igual, M.M.; Camacho, M.M.; Martínez-Navarrete, N. Effect of Process Technology on the Nutritional, Functional, and Physical Quality of Grapefruit Powder. Food Sci. Technol. Int. 2016, 23, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, VA, USA, 1990; pp. 931–935. [Google Scholar]
- Contreras, C.; Martín-Esparza, M.E.; Chiralt, A.; Martínez-Navarrete, N. Influence of Microwave Application on Convective Drying: Effects on Drying Kinetics, and Optical and Mechanical Properties of Apple and Strawberry. J. Food Eng. 2008, 88, 55–64. [Google Scholar] [CrossRef]
- Hutchings, J.B. Food Colour and Appearance; Blackie Academic & Professional: Glasglow, UK, 1999; pp. 199–235. [Google Scholar]
- Okos, M.R. Physical and Chemical Properties of Food; American Society of Agricultural Engineers: Saint Joseph, MI, USA, 1986; pp. 35–77. [Google Scholar]
- Tomás-Barberán, F.A.; Gil, M.; Cremin, P.; Waterhouse, A.L.; Hess-Pierce, B.; Kader, A.A. HPLC-DAD-ESIMS Analysis of Phenolic Compounds in Nectarines, Peaches, and Plums. J. Agric. Food Chem. 2001, 49, 4748–4760. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. Ferric Reducing/Antioxidant Power Assay: Direct Measure of Total Antioxidant Activity of Biological Fluids and Modified Version for Simultaneous Measurement of Total Antioxidant Power and Ascorbic Acid Concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Selvendran, R.R.; Ryden, P. Methods in Plant Biochemistry. In Carbohydrates; Dey, P.M., Ed.; Academic Press: London, UK, 1990; p. 549. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Sánchez-Mata, M.C.; Cámara-Hurtado, M.; Díez-Marqués, C.; Torija-Isasa, M.E. Comparison of High-Performance Liquid Chromatography and Spectrofluorimetry for Vitamin C Analysis of Green Beans (Phaseolus vulgaris, L.). Eur. Food Res. Technol. 2000, 210, 220–225. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Plaza, L.; De Ancos, B.; Cano, M.P. Quantitative Bioactive Compounds Assessment and Their Relative Contribution to the Antioxidant Capacity of Commercial Orange Juices. J. Sci. Food Agric. 2003, 83, 430–439. [Google Scholar] [CrossRef]
- Xu, B.J.; Chang, S.K.C. A Comparative Study on Phenolic Profiles and Antioxidant Activities of Legumes as Affected by Extraction Solvents. J. Food Sci. 2007, 72, 159–166. [Google Scholar] [CrossRef]
- Olives, A.I.; Cámara, M.; Sánchez, M.C.; Fernández, V.; López, M. Application of a UV-Vis Detection-HPLC Method for a Rapid Determination of Lycopene and β-Carotene in Vegetables. Food Chem. 2006, 95, 328–336. [Google Scholar] [CrossRef]
- Bodart, M.; de Peñaranda, R.; Deneyer, A.; Flamant, G. Photometry and Colorimetry Characterisation of Materials in Daylighting Evaluation Tools. Build. Environ. 2008, 43, 2046–2058. [Google Scholar] [CrossRef]
- Obied, H.K.; Bedgood, D.R.; Prenzler, P.D.; Robards, K. Effect of Processing Conditions, Prestorage Treatment, and Storage Conditions on the Phenol Content and Antioxidant Activity of Olive Mill Waste. J. Agric. Food Chem. 2008, 56, 3925–3932. [Google Scholar] [CrossRef]
- Igual, M.; García-Martínez, E.; Camacho, M.M.; Martínez-Navarrete, N. Effect of Thermal Treatment and Storage on the Stability of Organic Acids and the Functional Value of Grapefruit Juice. Food Chem. 2010, 118, 291–299. [Google Scholar] [CrossRef]
- Lin, T.M.; Durance, T.D.; Scaman, C.H. Characterization of Vacuum Microwave, Air and Freeze Dried Carrot Slices. Food Res. Int. 1998, 31, 111–117. [Google Scholar] [CrossRef]
- Fisher, J.F.; Rouseff, R.L. Solid-Phase Extraction and HPLC Determination of β-Cryptoxanthin and α- and β-Carotene in Orange Juice. J. Agric. Food Chem. 1986, 34, 985–989. [Google Scholar] [CrossRef]
- Tavarini, S.; Degl’Innocenti, E.; Remorini, D.; Massai, R.; Guidi, L. Antioxidant Capacity, Ascorbic Acid, Total Phenols and Carotenoids Changes during Harvest and after Storage of Hayward Kiwifruit. Food Chem. 2008, 107, 282–288. [Google Scholar] [CrossRef]






| Sample Code | Shelf Temperature (T) | Pressure (P) | Freezing Rate (FR) | ||||
|---|---|---|---|---|---|---|---|
| 30 °C | 40 °C | 50 °C | 5 Pa (P5) | 100 Pa (P100) | Slow (S) | Fast (F) | |
| S_30_P5 | X | X | X | ||||
| F_30_P5 | X | X | X | ||||
| S_30_P100 | X | X | X | ||||
| F_30_P100 | X | X | X | ||||
| S_40_P5 | X | X | X | ||||
| F_40_P5 | X | X | X | ||||
| S_40_P100 | X | X | X | ||||
| F_40_P100 | X | X | X | ||||
| S_50_P5 | X | X | X | ||||
| F_50_P5 | X | X | X | ||||
| S_50_P100 | X | X | X | ||||
| F_50_P100 | X | X | X | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Espinoza, M.A.; Ayed, C.; Foster, T.; Camacho, M.d.M.; Martínez-Navarrete, N. The Impact of Freeze-Drying Conditions on the Physico-Chemical Properties and Bioactive Compounds of a Freeze-Dried Orange Puree. Foods 2020, 9, 32. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9010032
Silva-Espinoza MA, Ayed C, Foster T, Camacho MdM, Martínez-Navarrete N. The Impact of Freeze-Drying Conditions on the Physico-Chemical Properties and Bioactive Compounds of a Freeze-Dried Orange Puree. Foods. 2020; 9(1):32. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9010032
Chicago/Turabian StyleSilva-Espinoza, Marilú A., Charfedinne Ayed, Timothy Foster, María del Mar Camacho, and Nuria Martínez-Navarrete. 2020. "The Impact of Freeze-Drying Conditions on the Physico-Chemical Properties and Bioactive Compounds of a Freeze-Dried Orange Puree" Foods 9, no. 1: 32. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9010032