Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review
Abstract
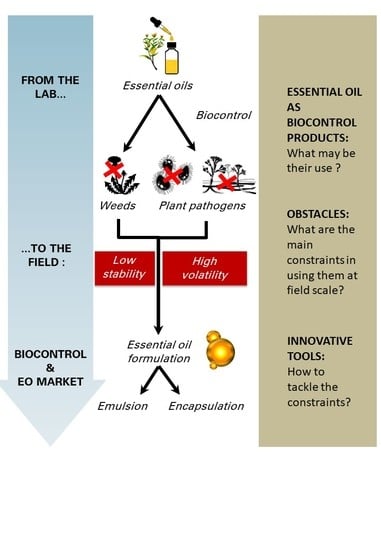
:1. Introduction
1.1. Specificities of Essential Oils
1.2. Essential Oil’s Use in Agriculture, against Plant Pathogens and Weeds
1.2.1. Antifungal and Anti-Oomycete Properties
1.2.2. Bactericidal Properties
1.2.3. Herbicidal Properties
1.2.4. Essential Oil’s Mechanisms of Action
- Inhibiting the fungi cell wall formation;
- Disrupting the cell membrane by inhibiting ergosterol synthesis;
- Affecting the fungal mitochondria by inhibiting the mitochondrial electron transport;
- Inhibiting cell division;
- Interfering with either RNA or DNA synthesis and/or inhibiting protein synthesis;
- Inhibiting efflux pumps.
- Mitosis inhibition;
- Decrease of the cellular respiration;
- Ion leakage and membrane depolarisation;
- Waxy cuticular layer removal;
- Decrease of the chlorophyll content;
- Oxidative damages through reactive oxygen species’ production;
- Microtubule polymerisation.
1.3. Market and Regulation
1.4. Innovative Avenue—Essential Oil Formulation
1.4.1. Essential Oils Emulsification
1.4.2. Essential Oils Encapsulation
2. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hancock, R.D.; Hogenhout, S.; Foyer, C.H. Mechanisms of plant-insect interaction. J. Exp. Bot. 2015, 66, 421–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, H.A.E.; El-Ghorab, A.H.; Shibamoto, T. Bioactivity of essential oils and their volatile aroma components: Review. J. Essent. Oil Res. 2012, 24, 203–212. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Van de Braak, S.A.A.J.; Leijten, G.C.J.J. Essential Oils and Oleoresins: A Survey in the Netherlands and Other Major Markets in the European Union; CBI, Centre for the Promotion of Imports from Developing Countries: Rotterdam, The Netherlands, 1999; p. 116. [Google Scholar]
- Koul, O.; Walia, S.; Dhaliwal, G.S. Essential oils as green pesticides: Potential and constraints. Biopestic. Int. 2008, 4, 63–84. [Google Scholar]
- Ravensberg, W. Crop protection in 2030: Towards a natural, efficient, safe and sustainable approach. In Proceedings of the IBMA International Symposium, Swansea University, Swansea, Wales, 7–9 September 2015. [Google Scholar]
- Lamichhane, J.R.; Dachbrodt-Saaydeh, S.; Kudsk, P.; Messéan, A. Toward a Reduced Reliance on Conventional Pesticides in European Agriculture. Plant Dis. 2016, 100, 10–24. [Google Scholar] [CrossRef] [Green Version]
- Barzman, M.; Bàrberi, P.; Birch, A.N.E.; Boonekamp, P.; Dachbrodt-Saaydeh, S.; Graf, B.; Hommel, B.; Jensen, J.E.; Kiss, J.; Kudsk, P.; et al. Eight principles of integrated pest management. Agron. Sustain. Dev. 2015, 35, 1199–1215. [Google Scholar] [CrossRef]
- Saenz-de-Cabezon, F.J.; Zalom, G.; Lopez-Olguin, F. A Review of Recent Patents on Macroorganisms as Biological Control Agents. Recent Pat. Biotechnol. 2010, 4, 48–64. [Google Scholar]
- Villaverde, J.J.; Sevilla-Morán, B.; Sandín-España, P.; López-Goti, C.; Alonso-Prados, J.L. Biopesticides in the framework of the European Pesticide Regulation (EC) No. 1107/2009. Pest Manag. Sci. 2014, 70, 2–5. [Google Scholar] [CrossRef]
- Khater, H.F. Prospects of Botanical Biopesticides in Insect Pest Management. Pharmacologia 2012, 3, 641–656. [Google Scholar]
- Isman, M.B. Pesticides Based on Plant Essential Oils: Phytochemical and Practical Considerations. In ACS Symposium Series; Jeliazkov (Zheljazkov), V.D., Cantrell, C.L., Eds.; American Chemical Society: Washington, DC, USA, 2016; pp. 13–26. [Google Scholar]
- Mossa, A.-T.H. Green Pesticides: Essential Oils as Biopesticides in Insect-pest Management. J. Environ. Sci. Technol. 2016, 9, 354–378. [Google Scholar] [CrossRef] [Green Version]
- Fierascu, R.C.; Fierascu, I.C.; Dinu-Pirvu, C.E.; Fierascu, I.; Paunescu, A. The application of essential oils as a next-generation of pesticides: Recent developments and future perspectives. Z. Für Nat. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ikbal, C.; Pavela, R. Essential oils as active ingredients of botanical insecticides against aphids. J. Pest Sci. 2019, 92, 971–986. [Google Scholar] [CrossRef]
- Isman, M.B. Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem. Rev. 2019, 1–7. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical Insecticides in the Twenty-First Century—Fulfilling Their Promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef] [Green Version]
- Isamn, M.B.; Tak, J.-H. Commercialization of insecticides based on plant essential oils: Past, present and future. In Green Pesticides Handbook: Essential Oils for Pest Contro; CRC Press: Boca Raton, FL, USA, 2017; pp. 27–39. [Google Scholar]
- Moharramipour, S.; Negahban, M. Plant Essential Oils and Pest Management. In Basic and Applied Aspects of Biopesticides; Sahayaraj, K., Ed.; Springer: New Delhi, India, 2014; pp. 129–153. [Google Scholar]
- Polatoğlu, K.; Karakoç, Ö.C. Biologically Active Essential Oils against Stored Product Pests. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 39–59. [Google Scholar]
- Carrubba, A.; Catalano, C. Essential Oil Crops for Sustainable Agriculture—A Review. In Climate Change, Intercropping, Pest Control and Beneficial Microorganisms; Lichtfouse, E., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 137–187. [Google Scholar]
- Da Cruz Francisco, J.; Sivik, B. Solubility of three monoterpenes, their mixtures and eucalyptus leaf oils in dense carbon dioxide. J. Supercrit. Fluids 2002, 23, 11–19. [Google Scholar] [CrossRef]
- Amri, I.; Gargouri, S.; Hamrouni, L.; Hanana, M.; Fezzani, T.; Jamoussi, B. Chemical composition, phytotoxic and antifungal activities of Pinus pinea essential oil. J. Pest Sci. 2012, 85, 199–207. [Google Scholar] [CrossRef]
- Dzamic, A.; Sokovic, M.; Ristic, M.S.; Grujic-Jovanovic, S.; Vukojevic, J.; Marin, P.D. Chemical composition and antifungal activity of Origanum heracleoticum essential oil. Chem. Nat. Compd. 2008, 44, 659–660. [Google Scholar] [CrossRef]
- Asbahani, A.E.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; Mousadik, A.E.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Impact of different storage conditions on the quality of selected essential oils. Food Res. Int. 2012, 46, 341–353. [Google Scholar] [CrossRef]
- Buckle, J. Basic plant taxonomy, basic essential oil chemistry, extraction, biosynthesis, and analysis. In Clinical Aromatherapy; Barlow, J., Ed.; Churchill Livingstone: St. Louis, MI, USA, 2015; pp. 37–72. [Google Scholar]
- Guenther, E. The Essential Oils; Krieger Publishing Co: Malabar, FL, USA, 1972; Volume I. [Google Scholar]
- Santos-Gomes, P.C.; Fernandes-Ferreira, M. Organ- and Season-Dependent Variation in the Essential Oil Composition of Salvia officinalis L. Cultivated at Two Different Sites. J. Agric. Food Chem. 2001, 49, 2908–2916. [Google Scholar] [CrossRef] [PubMed]
- Bhat, G.; Rasool, S.; Shakeel-u-Rehman, Ganaie, M.; Qazi, P.H.; Shawl, A.S. Seasonal Variation in Chemical Composition, Antibacterial and Antioxidant Activities of the Essential Oil of Leaves of Salvia officinalis (Sage) from Kashmir, India. J. Essent. Oil Bear. Plants 2016, 19, 1129–1140. [Google Scholar] [CrossRef]
- Khaosaad, T.; Vierheilig, H.; Nell, M.; Zitterl-Eglseer, K.; Novak, J. Arbuscular mycorrhiza alter the concentration of essential oils in oregano (Origanum sp., Lamiaceae). Mycorrhiza 2006, 16, 443–446. [Google Scholar] [CrossRef]
- Zolfaghari, M.; Nazeri, V.; Sefidkon, F.; Rejali, F. Effect of arbuscular mycorrhizal fungi on plant growth and essential oil content and composition of Ocimum basilicum L. Iran. J. Plant Physiol 2012, 3, 643–650. [Google Scholar]
- Seow, Y.X.; Yeo, C.R.; Chung, H.L.; Yuk, H.-G. Plant Essential Oils as Active Antimicrobial Agents. Crit. Rev. Food Sci. Nutr. 2014, 54, 625–644. [Google Scholar] [CrossRef]
- Zhang, J.; An, M.; Wu, H.; Liu, D.L.; Stanton, R. Chemical composition of essential oils of four Eucalyptus species and their phytotoxicity on silverleaf nightshade (Solanum elaeagnifolium Cav.) in Australia. Plant Growth Regul. 2012, 68, 231–237. [Google Scholar] [CrossRef]
- Odak, I.; Lukic, T.; Talic, S. Impact of Storage Conditions on Alteration of Juniper and Immortelle Essential Oils. J. Essent. Oil Bear. Plants 2018, 21, 614–622. [Google Scholar] [CrossRef]
- Kfoury, M. Préparation, Caractérisation Physicochimique et Évaluation des Propriétés Biologiques de Complexes D’inclusion à Base de Cyclodextrines: Applications à des Principes Actifs de Type Phénylpropanoïdes. Ph.D. Thesis, Université du Littoral Côte d’Opale, Calais, France, 2015. [Google Scholar]
- Nguyen, H.; Campi, E.M.; Roy Jackson, W.; Patti, A.F. Effect of oxidative deterioration on flavour and aroma components of lemon oil. Food Chem. 2009, 112, 388–393. [Google Scholar] [CrossRef]
- McGraw, G.W.; Hemingway, R.W.; Ingram, L.L.; Canady, C.S.; McGraw, W.B. Thermal degradation of terpenes: Camphene, Δ3-carene, limonene, and α-terpinene. Environ. Sci. Technol. 1999, 33, 4029–4033. [Google Scholar] [CrossRef]
- Misharina, T.A.; Polshkov, A.N. Antioxidant properties of essential oils: Autoxidation of essential oils from laurel and fennel and of their mixtures with essential oil from coriander. Appl. Biochem. Microbiol. 2005, 41, 610–618. [Google Scholar] [CrossRef]
- Castro, H.T.; Martínez, J.R.; Stashenko, E. Anethole Isomerization and Dimerization Induced by Acid Sites or UV Irradiation. Molecules 2010, 15, 5012–5030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Christensson, J.B. ared, Matura, M.; Gruvberger, B.; Bruze, M.; Karlberg, A.-T. Linalool–a significant contact sensitizer after air exposure. Contact Dermat. 2010, 62, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. The Influence of Global Environmental Change on Infectious Disease Dynamics: Workshop Summary; The National Academies Press: Washington, DC, USA, 2014. [Google Scholar]
- Jain, A.; Sarsaiya, S.; Wu, Q.; Lu, Y.; Shi, J. A review of plant leaf fungal diseases and its environment speciation. Bioengineered 2019, 10, 409–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology: Top 10 fungal pathogens. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [Green Version]
- Tabassum, N. and Vidyasagar, G.M. Antifungal investigations on plant essential oils. A review. Int. J. Pharm. Pharm. Sci. 2013, 5, 10. [Google Scholar]
- Lazar, E.E.; Jobling, J.J.; Benkeblia, N. Postharvest disease management of horticultural produce using essential oils: Today’s prospects. Stewart Postharvest Rev. 2010, 6, 1–10. [Google Scholar]
- Aminifard, M.H.; Mohammadi, S. Essential oils to control Botrytis cinerea in vitro and in vivo on plum fruits. J. Sci. Food Agric. 2012, 93, 348–353. [Google Scholar] [CrossRef]
- Božik, M.; Císarová, M.; Tančinová, D.; Kouřimská, L.; Hleba, L.; Klouček, P. Selected essential oil vapours inhibit growth of Aspergillus spp. in oats with improved consumer acceptability. Ind. Crop. Prod. 2017, 98, 146–152. [Google Scholar] [CrossRef]
- Ortiz de Elguea-Culebras, G.; Sánchez-Vioque, R.; Santana-Méridas, O.; Herraiz-Peñalver, D.; Carmona, M.; Berruga, M.I. In vitro antifungal activity of residues from essential oil industry against Penicillium verrucosum, a common contaminant of ripening cheeses. LWT Food Sci. Technol. 2016, 73, 226–232. [Google Scholar] [CrossRef]
- Abdolahi, A.; Hassani, A.; Ghosta, Y.; Meshkatalsadat, M.H.; Shabani, R. Screening of antifungal properties of essential oils extracted from sweet basil, fennel, summer savory and thyme against postharvest phytopathogenic fungi. J. Food Saf. 2011, 31, 350–356. [Google Scholar] [CrossRef]
- Yangui, I.; Zouaoui Boutiti, M.; Boussaid, M.; Messaoud, C. Essential Oils of Myrtaceae Species Growing Wild in Tunisia: Chemical Variability and Antifungal Activity Against Biscogniauxia mediterranea, the Causative Agent of Charcoal Canker. Chem. Biodivers. 2017, 14, e1700058. [Google Scholar] [CrossRef] [PubMed]
- Nana, W.L.; Eke, P.; Fokom, R.; Bakanrga-Via, I.; Begoude, D.; Tchana, T.; Tchameni, N.S.; Kuate, J.; Menut, C.; Fekam Boyom, F. Antimicrobial Activity of Syzygium aromaticum and Zanthoxylum xanthoxyloides Essential Oils Against Phytophthora megakarya. J. Phytopathol. 2015, 163, 632–641. [Google Scholar] [CrossRef]
- Sharma, A.; Rajendran, S.; Srivastava, A.; Sharma, S.; Kundu, B. Antifungal activities of selected essential oils against Fusarium oxysporum f. sp. lycopersici 1322, with emphasis on Syzygium aromaticum essential oil. J. Biosci. Bioeng. 2017, 123, 308–313. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Islam, R.; Islam, R.; Jamal, A.H.M.; Parvin, T.; Rahman, A. Chemical composition and antifungal properties of the essential oil and various extracts of Mikania scandens (L.) Willd. Arab. J. Chem. 2017, 10, S2170–S2174. [Google Scholar] [CrossRef] [Green Version]
- Abdolahi, A.; Hassani, A.; Ghosta, Y.; Javadi, T.; Meshkatalsadat, M.H. Essential oils as control agents of postharvest Alternaria and Penicillium rots on tomato fruits. J. Food Saf. 2010, 30, 341–352. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Abdelgaleil, S.A.M. Composition and antimicrobial activity of essential oils isolated from Egyptian plants against plant pathogenic bacteria and fungi. Ind. Crop. Prod. 2014, 52, 776–782. [Google Scholar] [CrossRef]
- Moghaddam, M.; Taheri, P.; Pirbalouti, A.G.; Mehdizadeh, L. Chemical composition and antifungal activity of essential oil from the seed of Echinophora platyloba DC. against phytopathogens fungi by two different screening methods. LWT Food Sci. Technol. 2015, 61, 536–542. [Google Scholar] [CrossRef]
- Puškárová, A.; Bučková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sapper, M.; Wilcaso, P.; Santamarina, M.P.; Roselló, J.; Chiralt, A. Antifungal and functional properties of starch-gellan films containing thyme (Thymus zygis) essential oil. Food Control 2018, 92, 505–515. [Google Scholar] [CrossRef]
- Xu, S.; Yan, F.; Ni, Z.; Chen, Q.; Zhang, H.; Zheng, X. In vitro and in vivo control of Alternaria alternata in cherry tomato by essential oil from Laurus nobilis of Chinese origin. J. Sci. Food Agric. 2014, 94, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Dan, Y.; Liu, H.-Y.; Gao, W.-W.; Chen, S.-L. Activities of essential oils from Asarum heterotropoides var. mandshuricum against five phytopathogens. Crop. Prot. 2010, 29, 295–299. [Google Scholar]
- Fraternale, D.; Flamini, G.; Ricci, D. Essential oil composition of Angelica archangelica L. (Apiaceae) roots and its antifungal activity against plant pathogenic fungi. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2016, 150, 558–563. [Google Scholar]
- Kacem, N.; Roumy, V.; Duhal, N.; Merouane, F.; Neut, C.; Christen, P.; Hostettmann, K.; Rhouati, S. Chemical composition of the essential oil from Algerian Genista quadriflora Munby and determination of its antibacterial and antifungal activities. Ind. Crop. Prod. 2016, 90, 87–93. [Google Scholar] [CrossRef]
- Znini, M.; Cristofari, G.; Majidi, L.; Paolini, J.; Desjobert, J.M.; Costa, J. Essential oil composition and antifungal activity of Pulicaria mauritanica Coss., against postharvest phytopathogenic fungi in apples. LWT Food Sci. Technol. 2013, 54, 564–569. [Google Scholar] [CrossRef]
- Znini, M.; Cristofari, G.; Majidi, L.; El Harrak, A.; Paolini, J.; Costa, J. In vitro antifungal activity and chemical composition of Warionia saharae essential oil against 3 apple phytopathogenic fungi. Food Sci. Biotechnol. 2013, 22, 113–119. [Google Scholar] [CrossRef]
- Dimić, G.; Kocić-Tanackov, S.; Mojović, L.; Pejin, J. Antifungal Activity of Lemon Essential Oil, Coriander and Cinnamon Extracts on Foodborne Molds in Direct Contact and the Vapor Phase. J. Food Process. Preserv. 2015, 39, 1778–1787. [Google Scholar] [CrossRef]
- Camiletti, B.X.; Asensio, C.M.; Pecci, M.D.L.P.G.; Lucini, E.I. Natural Control of Corn Postharvest Fungi Aspergillus flavus and Penicillium sp. Using Essential Oils from Plants Grown in Argentina. J. Food Sci. 2014, 79, M2499–M2506. [Google Scholar] [CrossRef]
- Jahani, M.; Pira, M.; Aminifard, M.H. Antifungal effects of essential oils against Aspergillus niger in vitro and in vivo on pomegranate (Punica granatum) fruits. Sci. Hortic. 2020, 264, 109188. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Kong, W.; Zhao, G.; Yang, M. Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem. 2017, 220, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.; Aziz, S.; Hussain, H. GC-MS Analysis and Antifungal Activity of Essential oils of Angelica glauca, Plectranthus rugosus, and Valeriana wallichii. J. Essent. Oil Bear. Plants 2012, 15, 15–21. [Google Scholar] [CrossRef]
- Kedia, A.; Dwivedy, A.K.; Jha, D.K.; Dubey, N.K. Efficacy of Mentha spicata essential oil in suppression of Aspergillus flavus and aflatoxin contamination in chickpea with particular emphasis to mode of antifungal action. Protoplasma 2016, 253, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Songsamoe, S.; Matan, N.; Matan, N. Antifungal activity of Michelia alba oil in the vapor phase and the synergistic effect of major essential oil components against Aspergillus flavus on brown rice. Food Control 2017, 77, 150–157. [Google Scholar] [CrossRef]
- Atif, M.; Ilavenil, S.; Devanesan, S.; AlSalhi, M.S.; Choi, K.C.; Vijayaraghavan, P.; Alfuraydi, A.A.; Alanazi, N.F. Essential oils of two medicinal plants and protective properties of jack fruits against the spoilage bacteria and fungi. Ind. Crop. Prod. 2020, 147, 112239. [Google Scholar] [CrossRef]
- Sonker, N.; Pandey, A.K.; Singh, P. Efficiency of Artemisia nilagirica (Clarke) Pamp. essential oil as a mycotoxicant against postharvest mycobiota of table grapes. J. Sci. Food Agric. 2015, 95, 1932–1939. [Google Scholar] [CrossRef] [PubMed]
- Bel Hadj Salah-Fatnassi, K.; Hassayoun, F.; Cheraif, I.; Khan, S.; Jannet, H.B.; Hammami, M.; Aouni, M.; Harzallah-Skhiri, F. Chemical composition, antibacterial and antifungal activities of flowerhead and root essential oils of Santolina chamaecyparissus L., growing wild in Tunisia. Saudi J. Biol. Sci. 2017, 24, 875–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanif, M.A.; Nawaz, H.; Ayub, M.A.; Tabassum, N.; Kanwal, N.; Rashid, N.; Saleem, M.; Ahmad, M. Evaluation of the effects of Zinc on the chemical composition and biological activity of basil essential oil by using Raman spectroscopy. Ind. Crop. Prod. 2017, 96, 91–101. [Google Scholar] [CrossRef]
- Nawaz, H.; Hanif, M.A.; Ayub, M.A.; Ishtiaq, F.; Kanwal, N.; Rashid, N.; Saleem, M.; Ahmad, M. Raman spectroscopy for the evaluation of the effects of different concentrations of Copper on the chemical composition and biological activity of basil essential oil. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 130–138. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Hoseini-Alfatemi, S.M.; Sharifi-Rad, M.; Setzer, W.N. Chemical Composition, Antifungal and Antibacterial Activities of Essential Oil from L allemantia Royleana (Benth. in Wall.) Benth. J. Food Saf. 2015, 35, 19–25. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Gruľová, D.; Baranová, B.; Caputo, L.; De Martino, L.; Sedlák, V.; Camele, I.; De Feo, V. Antimicrobial Activity and Chemical Composition of Essential Oil Extracted from Solidago canadensis L. Growing Wild in Slovakia. Molecules 2019, 24, 1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarai, Z.; Kadri, A.; Chobba, I.B.; Mansour, R.B.; Bekir, A.; Mejdoub, H.; Gharsallah, N. The in-vitro evaluation of antibacterial, antifungal and cytotoxic properties of Marrubium vulgare L. essential oil grown in Tunisia. Lipids Health Dis. 2011, 10, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Ghnaya, A.; Hanana, M.; Amri, I.; Balti, H.; Gargouri, S.; Jamoussi, B.; Hamrouni, L. Chemical composition of Eucalyptus erythrocorys essential oils and evaluation of their herbicidal and antifungal activities. J. Pest Sci. 2013, 86, 571–577. [Google Scholar] [CrossRef]
- Al-Reza, S.M.; Rahman, A.; Ahmed, Y.; Kang, S.C. Inhibition of plant pathogens in vitro and in vivo with essential oil and organic extracts of Cestrum nocturnum L. Pestic. Biochem. Physiol. 2010, 96, 86–92. [Google Scholar] [CrossRef]
- Montenegro, I.; Said, B.; Godoy, P.; Besoain, X.; Parra, C.; Díaz, K.; Madrid, A. Antifungal Activity of Essential Oil and Main Components from Mentha pulegium Growing Wild on the Chilean Central Coast. Agronomy 2020, 10, 254. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, V.K.; Cho, M.J.; Kang, S.C. Control of Plant Pathogenic Bacteria of Xanthomonas spp. by the Essential Oil and Extracts of Metasequoia glyptostroboides Miki ex Hu In vitro and In Vivo. J. Phytopathol. 2010, 158, 479–486. [Google Scholar] [CrossRef]
- Della Pepa, T.; Elshafie, H.S.; Capasso, R.; De Feo, V.; Camele, I.; Nazzaro, F.; Scognamiglio, M.R.; Caputo, L. Antimicrobial and Phytotoxic Activity of Origanum heracleoticum and O. majorana Essential Oils Growing in Cilento (Southern Italy). Molecules 2019, 24, 2576. [Google Scholar] [CrossRef] [Green Version]
- Ben Ghnaya, A.; Amri, I.; Hanana, M.; Gargouri, S.; Jamoussi, B.; Romane, A.; Hamrouni, L. Tetraclinis articulata (Vahl.) Masters essential oil from Tunisia: Chemical characterization and herbicidal and antifungal activities assessment. Ind. Crop. Prod. 2016, 83, 113–117. [Google Scholar] [CrossRef]
- Maissa, B.J.; Walid, H. Antifungal activity of chemically different essential oils from wild Tunisian Thymus spp. Nat. Prod. Res. 2015, 29, 869–873. [Google Scholar] [CrossRef]
- El Ouadi, Y.; Manssouri, M.; Bouyanzer, A.; Majidi, L.; Bendaif, H.; Elmsellem, H.; Shariati, M.A.; Melhaoui, A.; Hammouti, B. Essential oil composition and antifungal activity of Melissa officinalis originating from north-Est Morocco, against postharvest phytopathogenic fungi in apples. Microb. Pathog. 2017, 107, 321–326. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Alsanius, B.W. Cassia oil for controlling plant and human pathogens on fresh strawberries. Food Control 2012, 28, 157–162. [Google Scholar] [CrossRef]
- Yu, D.; Wang, J.; Shao, X.; Xu, F.; Wang, H. Antifungal modes of action of tea tree oil and its two characteristic components against Botrytis cinerea. J. Appl. Microbiol. 2015, 119, 1253–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rguez, S.; Ben Slimene, I.; Abid, G.; Hammemi, M.; Kefi, A.; Elkahoui, S.; Ksouri, R.; Hamrouni Sellami, I.; Djébali, N. Tetraclinis articulata essential oil reduces Botrytis cinerea infections on tomato. Sci. Hortic. 2020, 266, 109291. [Google Scholar] [CrossRef]
- Pragadheesh, V.S.; Saroj, A.; Yadav, A.; Chanotiya, C.S.; Alam, M.; Samad, A. Chemical characterization and antifungal activity of Cinnamomum camphora essential oil. Ind. Crop. Prod. 2013, 49, 628–633. [Google Scholar] [CrossRef]
- Saroj, A.; Pragadheesh, V.S.; Palanivelu, Yadav, A.; Singh, S.C.; Samad, A.; Negi, A.S.; Chanotiya, C.S. Anti-phytopathogenic activity of Syzygium cumini essential oil, hydrocarbon fractions and its novel constituents. Ind. Crop. Prod. 2015, 74, 327–335. [Google Scholar] [CrossRef]
- Rahman, A.; Al-Reza, S.M.; Kang, S.C. Antifungal Activity of Essential Oil and Extracts of Piper chaba Hunter Against Phytopathogenic Fungi. J. Am. Oil Chem. Soc. 2011, 88, 573–579. [Google Scholar] [CrossRef]
- Ali, A.; Wee Pheng, T.; Mustafa, M.A. Application of lemongrass oil in vapour phase for the effective control of anthracnose of “Sekaki” papaya. J. Appl. Microbiol. 2015, 118, 1456–1464. [Google Scholar] [CrossRef]
- Villa-Ruano, N.; Pacheco-Hernández, Y.; Rubio-Rosas, E.; Lozoya-Gloria, E.; Mosso-González, C.; Ramón-Canul, L.G.; Cruz-Durán, R. Essential oil composition and biological/pharmacological properties of Salmea scandens (L.) DC. Food Control 2015, 57, 177–184. [Google Scholar] [CrossRef]
- Ben Kaab, S.; Rebey, I.B.; Hanafi, M.; Berhal, C.; Fauconnier, M.L.; De Clerck, C.; Ksouri, R.; Jijakli, H. Rosmarinus officinalis essential oil as an effective antifungal and herbicidal agent. Span. J. Agric. Res. 2019, 17, e1006. [Google Scholar] [CrossRef] [Green Version]
- Zabka, M.; Pavela, R.; Gabrielova-Slezakova, L. Promising antifungal effect of some Euro-Asiatic plants against dangerous pathogenic and toxinogenic fungi. J. Sci. Food Agric. 2011, 91, 492–497. [Google Scholar] [CrossRef]
- Xing-dong, L.; Hua-li, X. Antifungal activity of the essential oil of Zanthoxylum bungeanum and its major constituent on Fusarium sulphureum and dry rot of potato tubers. Phytoparasitica 2014, 42, 509–517. [Google Scholar] [CrossRef]
- Brado Avanço, G.; Dias Ferreira, F.; Bomfim, N.S.; Andréia de Souza Rodrigues dos Santos, P.; Peralta, R.M.; Brugnari, T.; Mallmann, C.A.; Abreu Filho, B.A.D.; Mikcha, J.M.G.; Machinski, M., Jr. Curcuma longa L. essential oil composition, antioxidant effect, and effect on Fusarium verticillioides and fumonisin production. Food Control 2017, 73, 806–813. [Google Scholar]
- Boubaker, H.; Karim, H.; El Hamdaoui, A.; Msanda, F.; Leach, D.; Bombarda, I.; Vanloot, P.; Abbad, A.; Boudyach, E.H.; Ait Ben Aoumar, A. Chemical characterization and antifungal activities of four Thymus species essential oils against postharvest fungal pathogens of citrus. Ind. Crop. Prod. 2016, 86, 95–101. [Google Scholar] [CrossRef]
- Alves, M.F.; Nizio, D.A.D.C.; Sampaio, T.S.; Nascimento, A.F.D.; Brito, F.D.A.; Melo, J.O.D.; Arrigoni-Blank, M.D.F.; Gagliardi, P.R.; Machado, S.M.F.; Blank, A.F. Myrcia lundiana Kiaersk native populations have different essential oil composition and antifungal activity against Lasiodiplodia theobromae. Ind. Crop. Prod. 2016, 85, 266–273. [Google Scholar] [CrossRef]
- Khaledi, N.; Taheri, P.; Tarighi, S. Antifungal activity of various essential oils against Rhizoctonia solani and Macrophomina phaseolina as major bean pathogens. J. Appl. Microbiol. 2015, 118, 704–717. [Google Scholar] [CrossRef]
- Ozcakmak, S.; Gul, O.; Dervisoglu, M.; Yilmaz, A.; Sagdic, O.; Arici, M. Comparison of the Effect of Some Essential Oils on the Growth of Penicillium verrucosum and its Ochratoxin A production. J. Food Process. Preserv. 2017, 41, e13006. [Google Scholar] [CrossRef]
- Chanprapai, P.; Chavasiri, W. Antimicrobial activity from Piper sarmentosum Roxb. against rice pathogenic bacteria and fungi. J. Integr. Agric. 2017, 16, 2513–2524. [Google Scholar] [CrossRef] [Green Version]
- Ma, B.-X.; Ban, X.-Q.; He, J.-S.; Huang, B.; Zeng, H.; Tian, J.; Chen, Y.-X.; Wang, Y.-W. Antifungal activity of Ziziphora clinopodioides Lam. essential oil against Sclerotinia sclerotiorum on rapeseed plants (Brassica campestris L.). Crop. Prot. 2016, 89, 289–295. [Google Scholar] [CrossRef]
- Varo, A.; Mulero-Aparicio, A.; Adem, M.; Roca, L.F.; Raya-Ortega, M.C.; López-Escudero, F.J.; Trapero, A. Screening water extracts and essential oils from Mediterranean plants against Verticillium dahliae in olive. Crop. Prot. 2017, 92, 168–175. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, T.; Guo, Z.; Zhang, L.; Mao, L.; Zhang, Y.; Jiang, H. Fumigation and contact activities of 18 plant essential oils on Villosiclava virens, the pathogenic fungus of rice false smut. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Messgo-Moumene, S.; Li, Y.; Bachir, K.; Houmani, Z.; Bouznad, Z.; Chemat, F. Antifungal power of citrus essential oils against potato late blight causative agent. J. Essent. Oil Res. 2015, 27, 169–176. [Google Scholar] [CrossRef]
- Thanh, V.M.; Bui, L.M.; Bach, L.G.; Nguyen, N.T.; Thi, H.L.; Hoang Thi, T.T. Origanum majorana L. Essential Oil-Associated Polymeric Nano Dendrimer for Antifungal Activity against Phytophthora infestans. Materials 2019, 12, 1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalcanti, F.R.; Resende, M.L.V.; Carvalho, C.P.S.; Silveira, J.A.G.; Oliveira, J.T.A. Induced defence responses and protective effects on tomato against Xanthomonas vesicatoria by an aqueous extract from Solanum lycocarpum infected with Crinipellis perniciosa. Biol. Control 2006, 39, 408–417. [Google Scholar] [CrossRef]
- Ji, G.H.; Wei, L.F.; He, Y.Q.; Wu, Y.P.; Bai, X.H. Biological control of rice bacterial blight by Lysobacter antibioticus strain 13-1. Biol. Control 2008, 45, 288–296. [Google Scholar] [CrossRef]
- Moghaddam, M.; Alymanesh, M.R.; Mehdizadeh, L.; Mirzaei, H.; Ghasemi Pirbalouti, A. Chemical composition and antibacterial activity of essential oil of Ocimum ciliatum, as a new source of methyl chavicol, against ten phytopathogens. Ind. Crop. Prod. 2014, 59, 144–148. [Google Scholar] [CrossRef]
- Salamci, E.; Kordali, S.; Kotan, R.; Cakir, A.; Kaya, Y. Chemical compositions, antimicrobial and herbicidal effects of essential oils isolated from Turkish Tanacetum aucheranum and Tanacetum chiliophyllum var. chiliophyllum. Biochem. Syst. Ecol. 2007, 35, 569–581. [Google Scholar] [CrossRef]
- Kotan, R.; Cakir, A.; Dadasoglu, F.; Aydin, T.; Cakmakci, R.; Ozer, H.; Kordali, S.; Mete, E.; Dikbas, N. Antibacterial activities of essential oils and extracts of Turkish Achillea, Satureja and Thymus species against plant pathogenic bacteria. J. Sci. Food Agric. 2010, 90, 145–160. [Google Scholar] [CrossRef]
- Kotan, R.; Cakir, A.; Ozer, H.; Kordali, S.; Cakmakci, R.; Dadasoglu, F.; Dikbas, N.; Aydin, T.; Kazaz, C. Antibacterial effects of Origanum onites against phytopathogenic bacteria: Possible use of the extracts from protection of disease caused by some phytopathogenic bacteria. Sci. Hortic. 2014, 172, 210–220. [Google Scholar] [CrossRef]
- Kotan, R.; Dadasoğlu, F.; Karagoz, K.; Cakir, A.; Ozer, H.; Kordali, S.; Cakmakci, R.; Dikbas, N. Antibacterial activity of the essential oil and extracts of Satureja hortensis against plant pathogenic bacteria and their potential use as seed disinfectants. Sci. Hortic. 2013, 153, 34–41. [Google Scholar] [CrossRef]
- Okla, M.K.; Alamri, S.A.; Salem, M.Z.M.; Ali, H.M.; Behiry, S.I.; Nasser, R.A.; Alaraidh, I.A.; Al-Ghtani, S.M.; Soufan, W. Yield, Phytochemical Constituents, and Antibacterial Activity of Essential Oils from the Leaves/Twigs, Branches, Branch Wood, and Branch Bark of Sour Orange (Citrus aurantium L.). Processes 2019, 7, 363. [Google Scholar] [CrossRef] [Green Version]
- Luciardi, M.C.; Blázquez, M.A.; Cartagena, E.; Bardón, A.; Arena, M.E. Mandarin essential oils inhibit quorum sensing and virulence factors of Pseudomonas aeruginosa. LWT Food Sci. Technol. 2016, 68, 373–380. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Dung, N.T.; Suh, H.-J.; Kang, S.C. Antibacterial Activity of Essential Oil and Extracts of Cleistocalyx operculatus Buds Against the Bacteria of Xanthomonas spp. J. Am. Oil Chem. Soc. 2010, 87, 1341–1349. [Google Scholar] [CrossRef]
- Alkan, D.; Yemenicioğlu, A. Potential application of natural phenolic antimicrobials and edible film technology against bacterial plant pathogens. Food Hydrocoll. 2016, 55, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Behiry, S.I.; EL-Hefny, M.; Salem, M.Z.M. Toxicity effects of Eriocephalus africanus L. leaf essential oil against some molecularly identified phytopathogenic bacterial strains. Nat. Prod. Res. 2019, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Carezzano, M.E.; Sotelo, J.P.; Primo, E.; Reinoso, E.B.; Paletti Rovey, M.F.; Demo, M.S.; Giordano, W.F.; Oliva, M. de las M. Inhibitory effect of Thymus vulgaris and Origanum vulgare essential oils on virulence factors of phytopathogenic Pseudomonas syringae strains. Plant Biol. 2017, 19, 599–607. [Google Scholar] [CrossRef]
- Oliva, M.D.L.M.; Carezzano, M.E.; Giuliano, M.; Daghero, J.; Zygadlo, J.; Bogino, P.; Giordano, W.; Demo, M. Antimicrobial activity of essential oils of Thymus vulgaris and Origanum vulgare on phytopathogenic strains isolated from soybean. Plant Biol. 2015, 17, 758–765. [Google Scholar] [CrossRef]
- Amini, L.; Soudi, M.R.; Saboora, A.; Mobasheri, H. Effect of essential oil from Zataria multiflora on local strains of Xanthomonas campestris: An efficient antimicrobial agent for decontamination of seeds of Brassica oleracea var. capitata. Sci. Hortic. 2018, 236, 256–264. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Tarighi, S.; Taheri, P. Effects of plant essential oils on growth and virulence factors of Erwinia amylovora. J. Plant Pathol. 2019, 1–11. [Google Scholar] [CrossRef]
- Alipour, M.; Saharkhiz, M.J.; Niakousari, M.; Seidi Damyeh, M. Phytotoxicity of encapsulated essential oil of rosemary on germination and morphophysiological features of amaranth and radish seedlings. Sci. Hortic. 2019, 243, 131–139. [Google Scholar] [CrossRef]
- Fagodia, S.K.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Phytotoxicity and cytotoxicity of Citrus aurantiifolia essential oil and its major constituents: Limonene and citral. Ind. Crop. Prod. 2017, 108, 708–715. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, H.P.; Mittal, S.; Batish, D.R.; Kohli, R.K. Phytotoxic effects of volatile oil from Artemisia scoparia against weeds and its possible use as a bioherbicide. Ind. Crop. Prod. 2010, 32, 54–61. [Google Scholar] [CrossRef]
- Amri, I.; Hamrouni, L.; Hanana, M.; Jamoussi, B. Reviews on phytotoxic effects of essential oils and their individual components: News approach for weeds management. Int. J. Appl. Biol. Pharm. Technol. 2013, 4, 96–114. [Google Scholar]
- Blázquez, M. Role of Natural Essential Oils in Sustainable Agriculture and Food Preservation. JSRR 2014, 3, 1843–1860. [Google Scholar]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar] [CrossRef]
- De Almeida, L.F.R.; Frei, F.; Mancini, E.; De Martino, L.; De Feo, V. Phytotoxic Activities of Mediterranean Essential Oils. Molecules 2010, 15, 4309–4323. [Google Scholar] [CrossRef] [Green Version]
- Kordali, S.; Cakir, A.; Akcin, T.A.; Mete, E.; Akcin, A.; Aydin, T.; Kilic, H. Antifungal and herbicidal properties of essential oils and n-hexane extracts of Achillea gypsicola Hub-Mor. and Achillea biebersteinii Afan. (Asteraceae). Ind. Crop. Prod. 2009, 29, 562–570. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Setia, N.; Kohli, R.K. Herbicidal activity of volatile oils from Eucalyptus citriodora against Parthenium hysterophorus. Ann. Appl. Biol. 2005, 146, 89–94. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Setia, N.; Kohli, R.K.; Kaur, S.; Yadav, S.S. Alternative control of littleseed canary grass using eucalypt oil. Agron. Sustain. Dev. 2007, 27, 171–177. [Google Scholar] [CrossRef]
- Blázquez, M.A.; Carbó, E. Control of Portulaca oleracea by boldo and lemon essential oils in different soils. Ind. Crop. Prod. 2015, 76, 515–521. [Google Scholar] [CrossRef]
- Sumalan, R.M.; Alexa, E.; Popescu, I.; Negrea, M.; Radulov, I.; Obistioiu, D.; Cocan, I. Exploring Ecological Alternatives for Crop Protection Using Coriandrum sativum Essential Oil. Molecules 2019, 24, 2040. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Wu, H.; Feng, Y.; Deng, S.; Hou, A.; Che, F.; Liu, Y.; Geng, Q.; Ni, H.; Wei, Y. A strategy of rapidly screening out herbicidal chemicals from Eucalyptus essential oils. Pest Manag. Sci. 2020, 76, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, M.D.; Blázquez, M.A. Phytotoxic Effects of Commercial Eucalyptus citriodora, Lavandula angustifolia, and Pinus sylvestris Essential Oils on Weeds, Crops, and Invasive Species. Molecules 2019, 24, 2847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haig, T.J.; Haig, T.J.; Seal, A.N.; Pratley, J.E.; An, M.; Wu, H. Lavender as a Source of Novel Plant Compounds for the Development of a Natural Herbicide. J. Chem. Ecol. 2009, 35, 1129–1136. [Google Scholar] [CrossRef]
- Gruľová, D.; Caputo, L.; Elshafie, H.S.; Baranová, B.; De Martino, L.; Sedlák, V.; Gogaľová, Z.; Poráčová, J.; Camele, I.; De Feo, V. Thymol Chemotype Origanum vulgare L. Essential Oil as a Potential Selective Bio-Based Herbicide on Monocot Plant Species. Molecules 2020, 25, 595. [Google Scholar]
- Frabboni, L.; Tarantino, A.; Petruzzi, F.; Disciglio, G. Bio-Herbicidal Effects of Oregano and Rosemary Essential Oils on Chamomile (Matricaria chamomilla L.) Crop in Organic Farming System. Agronomy 2019, 9, 475. [Google Scholar] [CrossRef] [Green Version]
- Amri, I.; Hanana, M.; Jamoussi, B.; Hamrouni, L. Essential oils of Pinus nigra J.F. Arnold subsp. laricio Maire: Chemical composition and study of their herbicidal potential. Arab. J. Chem. 2017, 10, S3877–S3882. [Google Scholar] [CrossRef]
- Bainard, L.D.; Isman, M.B.; Upadhyaya, M.K. Phytotoxicity of clove oil and its primary constituent eugenol and the role of leaf epicuticular wax in the susceptibility to these essential oils. Weed Sci. 2006, 54, 833–837. [Google Scholar] [CrossRef]
- Laosinwattana, C.; Wichittrakarn, P.; Teerarak, M. Chemical composition and herbicidal action of essential oil from Tagetes erecta L. leaves. Ind. Crop. Prod. 2018, 126, 129–134. [Google Scholar] [CrossRef]
- Tworkoski, T. Herbicide effects of essential oils. Weed Sci. 2002, 50, 425–431. [Google Scholar]
- Lagrouh, F.; Dakka, N.; Bakri, Y. The antifungal activity of Moroccan plants and the mechanism of action of secondary metabolites from plants. J. Mycol. Méd. 2017, 27, 303–311. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-álvarez, J.A. Antifungal activities of thyme, clove and oregano essential oils. J. Food Saf. 2007, 27, 91–101. [Google Scholar] [CrossRef]
- Marzoug, H.N.B.; Romdhane, M.; Lebrihi, A.; Mathieu, F.; Couderc, F.; Abderraba, M.; Khouja, M.L.; Bouajila, J. Eucalyptus oleosa Essential Oils: Chemical Composition and Antimicrobial and Antioxidant Activities of the Oils from Different Plant Parts (Stems, Leaves, Flowers and Fruits). Molecules 2011, 16, 1695–1709. [Google Scholar] [CrossRef] [PubMed]
- Freires, I.d.A.; Murata, R.M.; Furletti, V.F.; Sartoratto, A.; Alencar, S.M.d.; Figueira, G.M.; de Oliveira Rodrigues, J.A.; Duarte, M.C.T.; Rosalen, P.L. Coriandrum sativum L. (Coriander) Essential Oil: Antifungal Activity and Mode of Action on Candida spp., and Molecular Targets Affected in Human Whole-Genome Expression. PLoS ONE 2014, 9, e99086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, F.; Ferreira, S.; Queiroz, J.A.; Domingues, F.C. Coriander (Coriandrum sativum L.) essential oil: Its antibacterial activity and mode of action evaluated by flow cytometry. J. Med Microbiol. 2011, 60, 1479–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, F.; Ferreira, S.; Duarte, A.; Mendonça, D.I.; Domingues, F.C. Antifungal activity of Coriandrum sativum essential oil, its mode of action against Candida species and potential synergism with amphotericin B. Phytomedicine 2011, 19, 42–47. [Google Scholar] [CrossRef]
- Hector, R.F. Compounds active against cell walls of medically important fungi. Clin. Microbiol. Rev. 1993, 6, 1–21. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Ramage, G.; Milligan, S.; Lappin, D.F.; Sherry, L.; Sweeney, P.; Williams, C.; Bagg, J.; Culshaw, S. Anti-fungal, cytotoxic, and immunomodulatory properties of tea tree oil and its derivative components: Potential role in management of oral candidosis in cancer patients. Front. Microbiol. 2012, 3, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furletti, V.F.; Teixeira, I.P.; Obando-Pereda, G.; Mardegan, R.C.; Sartoratto, A.; Figueira, G.M.; Duarte, R.M.; Rehder, V.L.; Duarte, M.C.; Höfling, J.F. Action of Coriandrum sativum L. essential oil upon oral Candida albicans biofilm formation. Evid. Based Complement. Altern. Med. 2011, 2011, 985832. [Google Scholar]
- Liu, R.H.; Shang, Z.C.; Yang, M.H.; Kong, L.Y. In vitro antibiofilm activity of Eucarobustol E against Candida albicans. Antimicrob. Agents Chemother. 2017, 61, 2707–2716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, M.; Henriques, M.; Azeredo, J.; Rocha, S.M.; Coimbra, M.A.; Oliveira, R. Morphogenesis control in Candida albicans and Candida dubliniensis through signaling molecules produced by planktonic and biofilm cells. Eukariot. Cell 2007, 6, 2429–2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ankri, S.; Mirelman, D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999, 1, 125–129. [Google Scholar] [CrossRef]
- Kurita, N.; Miyaji, M.; Kurane, R.; Takahara, Y. Antifungal Activity of Components of Essential Oils. Agric. Biol. Chem. 1981, 45, 945–952. [Google Scholar]
- Zuzarte, M.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Benzarti, A.; Marongiu, B.; Maxia, A.; Piras, A.; Salgueiro, L. Antifungal and anti-inflammatory potential of Lavandula stoechas and Thymus herba-barona essential oils. Ind. Crop. Prod. 2013, 44, 97–103. [Google Scholar] [CrossRef]
- Zabka, M.; Pavela, R. Antifungal efficacy of some natural phenolic compounds against significant pathogenic and toxinogenic filamentous fungi. Chemosphere 2013, 93, 1051–1056. [Google Scholar] [CrossRef]
- Dambolena, J.S.; López, A.G.; Meriles, J.M.; Rubinstein, H.R.; Zygadlo, J.A. Inhibitory effect of 10 natural phenolic compounds on Fusarium verticillioides. A structure–property–activity relationship study. Food Control 2012, 28, 163–170. [Google Scholar] [CrossRef]
- Cheng, S.-S.; Liu, J.-Y.; Chang, E.-H.; Chang, S.-T. Antifungal activity of cinnamaldehyde and eugenol congeners against wood-rot fungi. Bioresour. Technol. 2008, 99, 5145–5149. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Friedly, E.C.; Crandall, P.G.; Ricke, S.C.; Roman, M.; O’Bryan, C.; Chalova, V.I. In vitro Antilisterial Effects of Citrus Oil Fractions in Combination with Organic Acids. J. Food Sci. 2009, 74, M67–M72. [Google Scholar] [CrossRef]
- Chang, C.-W.; Chang, W.-L.; Chang, S.-T.; Cheng, S.-S. Antibacterial activities of plant essential oils against Legionella pneumophila. Water Res. 2008, 42, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Shukla, S.; Kang, S.C. Chemical composition and antifungal activity of essential oil and various extract of Silene armeria L. Bioresour. Technol. 2008, 99, 8903–8908. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Basti, A.A.; Misaghi, A.; Khaschabi, D. Growth response and modelling of the effects of Zataria multiflora Boiss. essential oil, pH and temperature on Salmonella Typhimurium and Staphylococcus aureus. LWT Food Sci. Technol. 2007, 40, 973–981. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. Antimicrobial activity of plant essential oils using food model media: Efficacy, synergistic potential and interactions with food components. Food Microbiol. 2009, 26, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Henika, P.R.; Levin, C.E.; Mandrell, R.E. Antibacterial Activities of Plant Essential Oils and Their Components against Escherichia coli O157:H7 and Salmonella enterica in Apple Juice. J. Agric. Food Chem. 2004, 52, 6042–6048. [Google Scholar] [CrossRef]
- Kotzekidou, P.; Giannakidis, P.; Boulamatsis, A. Antimicrobial activity of some plant extracts and essential oils against foodborne pathogens in vitro and on the fate of inoculated pathogens in chocolate. LWT Food Sci. Technol. 2008, 41, 119–127. [Google Scholar] [CrossRef]
- Canillac, N.; Mourey, A. Effects of several environmental factors on the anti-Listeria monocytogenes activity of an essential oil of Picea excelsa. Int. J. Food Microbiol. 2004, 92, 95–103. [Google Scholar] [CrossRef]
- Rivas, L.; McDonnell, M.J.; Burgess, C.M.; O’Brien, M.; Navarro-Villa, A.; Fanning, S.; Duffy, G. Inhibition of verocytotoxigenic Escherichia coli in model broth and rumen systems by carvacrol and thymol. Int. J. Food Microbiol. 2010, 139, 70–78. [Google Scholar] [CrossRef]
- Tassou, C.C.; Drosinos, E.H.; Nychas, G.J. Effects of essential oil from mint (Mentha piperita) on Salmonella enteritidis and Listeria monocytogenes in model food systems at 4 degrees and 10 degrees C. J. Appl. Bacteriol. 1995, 78, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Lins, L.; Dal Maso, S.; Foncoux, B.; Kamili, A.; Laurin, Y.; Genva, M.; Jijakli, M.H.; De Clerck, C.; Fauconnier, M.L.; Deleu, M. Insights into the Relationships Between Herbicide Activities, Molecular Structure and Membrane Interaction of Cinnamon and Citronella Essential Oils Components. IJMS 2019, 20, 4007. [Google Scholar] [CrossRef] [Green Version]
- Maffei, M.; Camusso, W.; Sacco, S. Effect of Mentha x piperita essential oil and monoterpenes on cucumber root membrane potential. Phytochemistry 2001, 58, 703–707. [Google Scholar] [CrossRef]
- Zunino, M.P.; Zygadlo, J.A. Effect of monoterpenes on lipid oxidation in maize. Planta 2004, 219, 303–309. [Google Scholar] [PubMed]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils: Science, Technology, and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The development, regulation and use of biopesticides for integrated pest management. Philos. Trans. R. Soc. B 2011, 366, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Czaja, K.; Goralczyk, K.; Struciński, P.; Hernik, A.; Korcz, W.; Minorczyk, M.; Łyczewska, M.; Ludwicki, J.K. Biopesticides - Towards increased consumer safety in the European Union. Pest Manag. Sci. 2015, 71, 3–6. [Google Scholar] [CrossRef]
- Rathore, H.S. Green Pesticides for Organic Farming: Occurrence and Properties of Essential Oils for Use in Pest Control. In Green Pesticides Handbook: Essential Oils for Pest Control; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2017; pp. 3–26. [Google Scholar]
- Siah, A.; Magnin-Robert, M.; Randoux, B.; Choma, C.; Rivière, C.; Halama, P.; Reignault, P. Natural Agents Inducing Plant Resistance Against Pests and Diseases. In Natural Antimicrobial Agents; Mérillon, J.-M., Riviere, C., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 121–159. [Google Scholar]
- Cary, D. Biological Control Methods, expected growth over the next 15 years and the key factors impacting their adoption. In Proceedings of the OECD Meeting, Brisbane, Australia, 2 December 2015. [Google Scholar]
- Dunham, W.C. Evolution and future of biocontrol. In Proceedings of the 10th Annual Biocontrol Industry Meeting (ABIM), Basel, Switzerland, 20 October 2015. [Google Scholar]
- Olson, S. An Analysis of the Biopesticide Market Now and Where it is Going. Outlooks Pest Manag. 2015, 26, 203–206. [Google Scholar] [CrossRef]
- Centre for the Promotion of Imports from Developing Countries. Available online: https://www.cbi.eu/market-information/natural-ingredients-cosmetics/essential-oils-fragrances/ (accessed on 15 April 2018).
- Grand View Research Database. Available online: https://www.grandviewresearch.com/industry-analysis/essential-oils-market/ (accessed on 10 March 2018).
- Mohan, M.; Haider, S.Z.; Andola, H.C.; Purohit, V.K. Essential Oils as Green Pesticides: For Sustainable Agriculture. Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 100–106. [Google Scholar]
- Quarles, W. EPA Exempts Least-Toxic Pesticides. IPM Practitioner 1996, 18, 16–17. [Google Scholar]
- Isman, M.B.; Miresmailli, S.; Machial, C. Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem. Rev. 2011, 10, 197–204. [Google Scholar] [CrossRef]
- European Chemical Agency. Available online: https://www.echa.europa.eu/ (accessed on 5 April 2018).
- Pascual-Villalobos, M.J.; Guirao, P.; Díaz-Baños, F.G.; Cantó-Tejero, M.; Villora, G. Oil in water nanoemulsion formulations of botanical active substances. In Nano-Biopesticides Today and Future Perspectives; Elsevier: Amsterdam, The Netherlands, 2019; pp. 223–247. [Google Scholar]
- Libs, E.; Salim, E. Formulation of Essential Oil Pesticides Technology and their Application. Agric. Res. Technol. 2017, 9, 555759. [Google Scholar] [CrossRef]
- Kfoury, M.; Lounès-Hadj Sahraoui, A.; Bourdon, N.; Laruelle, F.; Fontaine, J.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Solubility, photostability and antifungal activity of phenylpropanoids encapsulated in cyclodextrins. Food Chem. 2016, 196, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Pavoni, L.; Pavela, R.; Cespi, M.; Bonacucina, G.; Maggi, F.; Zeni, V.; Canale, A.; Lucchi, A.; Bruschi, F.; Benelli, G. Green Micro- and Nanoemulsions for Managing Parasites, Vectors and Pests. Nanomaterials 2019, 9, 1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aswathanarayan, J.B.; Vittal, R.R. Nanoemulsions and Their Potential Applications in Food Industry. Front. Sustain. Food Syst. 2019, 3, 95. [Google Scholar] [CrossRef] [Green Version]
- Komaiko, J.S.; McClements, D.J. Formation of Food-Grade Nanoemulsions Using Low-Energy Preparation Methods: A Review of Available Methods. Compr. Rev. Food Sci. Food Saf. 2016, 15, 331–352. [Google Scholar] [CrossRef]
- Anton, N.; Vandamme, T. Nano-emulsions and microemulsions: Clarification of the critical differences. Pharm. Res. 2011, 28, 978–985. [Google Scholar] [CrossRef]
- Naserzadeh, Y.; Naserzadeh, Y.; Mahmoudi, N.; Mahmoudi, N.; Pakina, E.; Pakina, E. Antipathogenic effects of emulsion and nanoemulsion of cinnamon essential oil against Rhizopus rot and grey mold on strawberry fruits. Foods Raw Mater. 2019, 7, 210–216. [Google Scholar] [CrossRef]
- Liang, R.; Xu, S.; Shoemaker, C.F.; Li, Y.; Zhong, F.; Huang, Q. Physical and antimicrobial properties of peppermint oil nanoemulsions. J. Agric. Food Chem. 2012, 60, 7548–7555. [Google Scholar] [CrossRef]
- Moretti, M.D.; Sanna-Passino, G.; Demontis, S.; Bazzoni, E. Essential oil formulations useful as a new tool for insect pest control. Aaps Pharmscitech 2002, 3, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Pavoni, L.; Maggi, F.; Mancianti, F.; Nardoni, S.; Ebani, V.V.; Cespi, M.; Bonacucina, G.; Palmieri, G.F. Microemulsions: An effective encapsulation tool to enhance the antimicrobial activity of selected EOs. J. Drug Deliv. Sci. Technol. 2019, 53, 101101. [Google Scholar] [CrossRef]
- Osman Mohamed Ali, E.; Shakil, N.A.; Rana, V.S.; Sarkar, D.J.; Majumder, S.; Kaushik, P.; Singh, B.B.; Kumar, J. Antifungal activity of nano emulsions of neem and citronella oils against phytopathogenic fungi, Rhizoctonia solani and Sclerotium rolfsii. Ind. Crop. Prod. 2017, 108, 379–387. [Google Scholar] [CrossRef]
- Sasson, Y.; Levy-Ruso, G.; Toledano, O.; Ishaaya, I. Nanosuspensions: Emerging novel agrochemical formulations. In Insecticides Design Using Advanced Technologies; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–39. [Google Scholar]
- Song, S.; Liu, X.; Jiang, J.; Qian, Y.; Zhang, N.; Wu, Q. Stability of triazophos in self-nanoemulsifying pesticide delivery system. Colloids Surf. A Physicochem. Eng. Asp. 2009, 350, 57–62. [Google Scholar] [CrossRef]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108, 303–318. [Google Scholar] [CrossRef]
- Borges, D.F.; Lopes, E.A.; Fialho Moraes, A.R.; Soares, M.S.; Visôtto, L.E.; Oliveira, C.R.; Moreira Valente, V.M. Formulation of botanicals for the control of plant-pathogens: A review. Crop. Prot. 2018, 110, 135–140. [Google Scholar] [CrossRef]
- Detoni, C.B.; de Oliveira, D.M.; Santo, I.E.; Pedro, A.S.; El-Bacha, R.; da Silva Velozo, E.; Ferreira, D.; Sarmento, B.; de Magalhães Cabral-Albuquerque, E.C. Evaluation of thermal-oxidative stability and antiglioma activity of Zanthoxylum tingoassuiba essential oil entrapped into multi- and unilamellar liposomes. J. Liposome Res. 2012, 22, 1–7. [Google Scholar] [CrossRef]
- Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Influence of emulsion composition and inlet air temperature on the microencapsulation of flaxseed oil by spray drying. Food Res. Int. 2011, 44, 282–289. [Google Scholar] [CrossRef]
- Martins, I.M.; Rodrigues, S.N.; Barreiro, F.; Rodrigues, A.E. Microencapsulation of thyme oil by coacervation. J. Microencapsul. 2009, 26, 667–675. [Google Scholar] [CrossRef]
- Piacentini, E.; Giorno, L.; Dragosavac, M.M.; Vladisavljević, G.T.; Holdich, R.G. Microencapsulation of oil droplets using cold water fish gelatine/gum arabic complex coacervation by membrane emulsification. Food Res. Int. 2013, 53, 362–372. [Google Scholar] [CrossRef] [Green Version]
- Ocak, B.; Gülümser, G.; Baloğlu, E. Microencapsulation of Melaleuca alternifolia (Tea Tree) Oil by Using Simple Coacervation Method. J. Essent. Oil Res. 2011, 23, 58–65. [Google Scholar] [CrossRef]
- Ciobanu, A.; Mallard, I.; Landy, D.; Brabie, G.; Nistor, D.; Fourmentin, S. Retention of aroma compounds from Mentha piperita essential oil by cyclodextrins and crosslinked cyclodextrin polymers. Food Chem. 2013, 138, 291–297. [Google Scholar] [CrossRef]
- Rakmai, J.; Cheirsilp, B.; Mejuto, J.C.; Torrado-Agrasar, A.; Simal-Gándara, J. Physico-chemical characterization and evaluation of bio-efficacies of black pepper essential oil encapsulated in hydroxypropyl-beta-cyclodextrin. Food Hydrocoll. 2017, 65, 157–164. [Google Scholar] [CrossRef]
- Varona, S.; Kareth, S.; Cocero, M.J. Encapsulation of essentials oils using biopolymers for their use in ecological agriculture. In Proceedings of the International Symposium on Supercritical Fluids, Arcachon, France, 18–20 May 2009. [Google Scholar]
- Varona, S.; Kareth, S.; Martín, Á.; Cocero, M.J. Formulation of lavandin essential oil with biopolymers by PGSS for application as biocide in ecological agriculture. J. Supercrit. Fluids 2010, 54, 369–377. [Google Scholar] [CrossRef]
- Gonçalves, N.D.; Pena, F.D.L.; Sartoratto, A.; Derlamelina, C.; Duarte, M.C.T.; Antunes, A.E.C.; Prata, A.S. Encapsulated thyme (Thymus vulgaris) essential oil used as a natural preservative in bakery product. Food Res. Int. 2017, 96, 154–160. [Google Scholar]
- Girardi, N.S.; García, D.; Passone, M.A.; Nesci, A.; Etcheverry, M. Microencapsulation of Lippia turbinata essential oil and its impact on peanut seed quality preservation. Int. Biodeterior. Biodegrad. 2017, 116, 227–233. [Google Scholar] [CrossRef]
- Girardi, N.S.; García, D.; Robledo, S.N.; Passone, M.A.; Nesci, A.; Etcheverry, M. Microencapsulation of Peumus boldus oil by complex coacervation to provide peanut seeds protection against fungal pathogens. Ind. Crop. Prod. 2016, 92, 93–101. [Google Scholar] [CrossRef]
- Yoshida, P.A.; Yokota, D.; Foglio, M.A.; Rodrigues, R.A.F.; Pinho, S.C. Liposomes incorporating essential oil of Brazilian cherry (Eugenia uniflora L.): Characterization of aqueous dispersions and lyophilized formulations. J. Microencapsul. 2010, 27, 416–425. [Google Scholar] [CrossRef]
- Lai, F.; Wissing, S.A.; Müller, R.H.; Fadda, A.M. Artemisia arborescens L essential oil-loaded solid lipid nanoparticles for potential agricultural application: Preparation and characterization. Aaps Pharmscitech 2006, 7, E10. [Google Scholar] [CrossRef] [Green Version]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Wissing, S.; Kayser, O.; Müller, R. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef]
- Maryam, I.; Huzaifa, U.; Hindatu, H.; Zubaida, S. Nanoencapsulation of essential oils with enhanced antimicrobial activity: A new way of combating antimicrobial Resistance. J. Pharmacogn. Phytochem. 2015, 4, 165. [Google Scholar]
- Mogul, M.G.; Akin, H.; Hasirci, N.; Trantolo, D.J.; Gresser, J.D.; Wise, D.L. Controlled release of biologically active agents for purposes of agricultural crop management. Resour. Conserv. Recycl. 1996, 16, 289–320. [Google Scholar] [CrossRef]
- De Oliveira, J.L.; Campos, E.V.R.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Application of nanotechnology for the encapsulation of botanical insecticides for sustainable agriculture: Prospects and promises. Biotechnol. Adv. 2014, 32, 1550–1561. [Google Scholar] [CrossRef]
- Isman, M.B.; Grieneisen, M.L. Botanical insecticide research: Many publications, limited useful data. Trends Plant Sci. 2014, 19, 140–145. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Kai Seng, K.; Voon Loong, W. Introductory Chapter: From Microemulsions to Nanoemulsions. Nanoemulsions—Properties. Fabr. Appl. 2019. [Google Scholar] [CrossRef] [Green Version]
- Lamichhane, J.R.; Bischoff-Schaefer, M.; Bluemel, S.; Dachbrodt-Saaydeh, S.; Dreux, L.; Jansen, J.-P.; Kiss, J.; Köhl, J.; Kudsk, P.; Malausa, T.; et al. Identifying obstacles and ranking common biological control research priorities for Europe to manage most economically important pests in arable, vegetable and perennial crops. Pest Manag. Sci. 2017, 73, 14–21. [Google Scholar] [CrossRef]
- Knight, S.; Hauxwell, J. Distribution and Abundance of Aquatic Plants—Human Impacts. In Encyclopedia of Inland Waters; Elsevier: Amsterdam, The Netherlands, 2009; pp. 45–54. [Google Scholar]
| Target Organism | Disease Caused by the Pathogen | Essential Oil Distilled from | References | |
|---|---|---|---|---|
| Fungi | Alternaria alternata | Leaf spot, alternariose | Carum carvi L., Carum opticum L. and Foeniculum vulgare L. | [58] |
| 18 egyptian plants | [59] | |||
| Echinophora platyloba (seed) | [60] | |||
| Thuja plicata, Eugenia caryophillata L., Lavandula angustifolia, Origanum vulgare L., Salvia sclarea and Thymus vulgaris L. | [61] | |||
| Thymus zygiis | [62] | |||
| Laurus nobilis | [63] | |||
| Alternaria humicola | Alternariose | Asarum heterotropoides | [64] | |
| Alternaria solani | Early blight | Angelica archangelica | [65] | |
| Alternaria spp. | Alternariose | Pinus pinea | [25] | |
| Genista quadriflora | [66] | |||
| Pulicaria mauritanica | [67] | |||
| Warionia saharae | [68] | |||
| Aspergillus carbonarius | Ochratoxin producer | Citrus x limon L. | [69] | |
| Aspergillus flavus | Rot and mould, aflatoxins production, aspergillosis | Mentha x piperita, Origanum spp., Rosmarinus officinalis L., Schinus mole L. and Tagetes minuta L. | [70] | |
| Eucalyptus sp., Ferula galbaniflua, Thymus capitatus and Syzygium aromaticum | [71] | |||
| Curcuma longa | [72] | |||
| Angelica glauca, Plectranthus rugosus, Valeriana wallichii | [73] | |||
| Mentha spicata | [74] | |||
| Michelia alba | [75] | |||
| Ocimum basilicum and Vetiveria zizanioides | [76] | |||
| Artemisia nilagirica | [77] | |||
| Aspergillus fumigatus | Rot and mould, aflatoxins production, aspergillosis | Santolina chamaecyparissus | [78] | |
| Thuja plicata, Eugenia caryophillata L., Lavandula angustifolia, Origanum vulgare L., Salvia sclarea and Thymus vulgaris L. | [61] | |||
| Aspergillus niger | Mould | Ocimum basilicum L. | [79] | |
| Genista quadriflora | [66] | |||
| Ocimum basilicum L. | [80] | |||
| Lallemantia royleana | [81] | |||
| Artemisia nilagirica | [77] | |||
| Ocimum basilicum and Vetiveria zizanioides | [76] | |||
| Solidago canadensis L. | [82] | |||
| Marrubium vulgare | [83] | |||
| Aspergillus ochraceus | Ochratoxin producer | Artemisia nilagirica | [77] | |
| Aspergillus parasiticus | Mould | Citrus x limon L. | [69] | |
| Aspergillus spp. | Cinnamomum zeylanicum, Thymus vulgaris L., Origanum vulgare L., Syzygium aromaticum L.), Cymbopogon citratus and Zingiber officinale Rosc. | [51] | ||
| 14 different botanical plant species | [72] | |||
| Bipolaris oryzae | Brown spot | Piper sarmentosum | [73] | |
| Fungi | Bipolaris sorokiniana | Leaf blight/spot | Pinus pinea | [25] |
| Eucalyptus erythrocorys | [84] | |||
| Biscogniauxia mediterranea | Charcoal disease | Eucalyptus spp. | [54] | |
| Botryotinia fuckeliana | Grey mould | Thymus zygiis | [62] | |
| Botrytis cinerea | Grey mould | Cestrum nocturnum | [85] | |
| Carum carvi L., Foeniculum vulgare L. and Mentha x piperita | [50] | |||
| 18 egyptian plants | [59] | |||
| Mentha pulegium | [86] | |||
| Metasequoia glyptostroboides | [87] | |||
| Origanum heracleoticum | [88] | |||
| Origanum majorana | ||||
| Eucalyptus erythrocorys | [84] | |||
| Tetraclinis articulata | [89] | |||
| Thymus spp. | [90] | |||
| Melissa officinalis | [91] | |||
| Cinnamomum cassia | [92] | |||
| Angelica archangelica | [65] | |||
| Solidago canadensis L. | [82] | |||
| Melaleuca alternifolia | [93] | |||
| Tetraclinis articulata | [94] | |||
| Marrubium vulgare | [83] | |||
| Choanephora cucurbitarum | Fruit and blossom rot | Cinnamomum camphora | [95] | |
| Syzygium cumini | [96] | |||
| Cladosporium cladosporioides | Rot | Citrus x limon L. | [69] | |
| Thuja plicata, Eugenia caryophillata L., Lavandula angustifolia, Origanum vulgare L., Salvia sclarea and Thymus vulgaris L. | [61] | |||
| Colletotrichum capsici | Leaf spot | Cestrum nocturnum | [85] | |
| Metasequoia glyptostroboides | [87] | |||
| Piper chaba | [97] | |||
| Colletotrichum gloeosporioides | Leaf spot | Cymbopogon sp. | [98] | |
| Asarum heterotropoides | [64] | |||
| Colletotrichum tricbellum | Leaf spot | Echinophora platyloba (seed) | [60] | |
| Curvularia fallax | Black sheath spot—Leaf spot | |||
| Cytospara sacchari | Stem canker on sugarcane | |||
| Eurotium herbariorum | Mould | Citrus x limon L. | [69] | |
| Fusarium avenaceum | Ear blight and root rot of cereals | Eucalyptus erythrocorys | [84] | |
| Fusarium oxysporum | Fusarium wilt (vascular disease) | 18 egyptian plant species | [59] | |
| Metasequoia glyptostroboides | [87] | |||
| Eucalyptus erythrocorys | [84] | |||
| Fungi | Fusarium oxysporum | Fusarium wilt (vascular disease) | Genista quadriflora | [66] |
| Echinophora platyloba (seed) | [60] | |||
| Piper chaba | [97] | |||
| Syzygium aromaticum, Eucalyptus globulus, Cymbopogon citratus and Mentha x piperita | [56] | |||
| Mikania scandens | [57] | |||
| Salmea scandens | [99] | |||
| Phytophthora megakarya | Black pod disease | Syzygium aromaticum and Zanthoxylum xanthoxyloides | [55] | |
| Pythium spp. | Root rot | Thymus spp. | [90] | |
| Mikania scandens | [57] | |||
| Fusarium solani | Root rot, soft rot of plant tissues | 18 egyptian plant species | [59] | |
| Metasequoia glyptostroboides | [87] | |||
| Eucalyptus erythrocorys | [84] | |||
| Asarum heterotropoides | [64] | |||
| Angelica glauca, Plectranthus rugosus, Valeriana wallichii | [73] | |||
| Piper chaba | [97] | |||
| Marrubium vulgare | [83] | |||
| Fusarium spp. | Cestrum nocturnum | [85] | ||
| Pinus pinea | [25] | |||
| Rosmarinus officinalis | [100] | |||
| Tetraclinis articulata | [90] | |||
| Angelica archangelica | [65] | |||
| 14 different botanical plant species | [101] | |||
| Fusarium sulphureum | Dry rot | Zanthoxylum bungeanum | [102] | |
| Fusarium verticillioides | Ear rot on maize | Curcuma longa | [103] | |
| Geotrichum citri-aurantii | Sour rot (post-harvest) | Thymus spp. | [104] | |
| Lasiodiplodia theobromae | Rot and dieback (forest species) | Myrcia lundiana | [105] | |
| Macrophomina phaseolina | Damping-off, seedling blight, rot | Mentha x piperita and Ocimum basilicum | [106] | |
| Echinophora platyloba (seed) | [60] | |||
| Microdochium nivale | Patch lawn disease | Pinus pinea | [25] | |
| Monilinia fructicola | Brown rot | Mentha pulegium | [86] | |
| Solidago canadensis L. | [82] | |||
| Penicillium digitatum | Green mould (post-harvest) | Carum carvi L., Carum opticum L. and Foeniculum vulgare L. | [58] | |
| Foeniculum vulgare Mill., Satureja hortensis L., Ocimum basilicum L. and Thymus vulgaris L. | [53] | |||
| Thymus spp. | [104] | |||
| Marrubium vulgare | [83] | |||
| Penicillium expansum | Post-harvest mould | Melissa officinalis | [91] | |
| Pulicaria mauritanica | [67] | |||
| Solidago canadensis L. | [82] | |||
| Warionia saharae | [68] | |||
| Fungi | Penicillium italicum | Blue mould | Thymus spp. | [104] |
| Rosmarinus officinalis | [100] | |||
| Penicillium spp. | Mentha x piperita, Origanum spp., Rosmarinus officinalis L., Schinus mole L. and Tagetes minuta L. | [70] | ||
| Citrus x limon L. | [69] | |||
| Ocimum basilicum | [79] | |||
| Ocimum basilicum | [80] | |||
| Ocimum basilicum and Vetiveria zizanioides | [76] | |||
| 14 different botanical plant species | [101] | |||
| Penicillium verrucosum | Ochratoxin producer | Residues of Lamiceae species | [52] | |
| Allium sativum L., Mentha x piperita, Origanum onites L. and Salvia officinalis L. | [107] | |||
| Rhizoctonia solani | Damping-off, root and stems rot | Cestrum nocturnum | [85] | |
| Metasequoia glyptostroboides | [87] | |||
| Asarum heterotropoides | [64] | |||
| Angelica archangelica | [65] | |||
| Bunium persicum, Foeniculum vulgare, Juniperus polycarpus, Mentha spp., Ocimum basilicum, Thymus vulgaris and Zingiber officinale | [106] | |||
| Piper chaba | [97] | |||
| Syzygium cumini | [96] | |||
| Thymus spp. | [90] | |||
| Mikania scandens | [57] | |||
| Piper sarmentosum | [108] | |||
| Rhizoctonia sp. | Pinus pinea | [25] | ||
| Rhizopus microsporus | Rice seedling blight, various head, grain and ear rots | Ocimum basilicum and Vetiveria zizanioides | [76] | |
| Rhizopus stolonifer | Storage/post-harvest rot | Foeniculum vulgare Mill., Satureja hortensis L., Ocimum basilicum L. and Thymus vulgaris L. | [53] | |
| Melissa officinalis | [91] | |||
| Pulicaria mauritanica | [67] | |||
| Warionia saharae | [68] | |||
| Sclerotinia sclerotiorum | White mould | Cestrum nocturnum | [85] | |
| Metasequoia glyptostroboides | [87] | |||
| Ziziphora clinopodioides | [109] | |||
| Verticillium dahliae | Verticillium wilt | 35 plant’s botanical species | [110] | |
| Villosiclava virens | Rice false smut | 18 plant’s botanical species | [111] | |
| Oomycetes | Phytophthora cactorum | Root rot | Asarum heterotropoides | [64] |
| Phytophthora capsici | Blight | Cestrum nocturnum | [85] | |
| Metasequoia glyptostroboides | [87] | |||
| Piper chaba | [97] | |||
| Phytophthora infestans | Late blight | Salmea scandens | [99] | |
| Citrus sinensis Cadenera, Citrus limon Eureka and Citrus bergamia Castagnaro | [112] | |||
| Thymus spp. | [90] | |||
| Origanum majorana L. | [113] | |||
| Oomycetes | Phytophthora megakarya | Black pod disease | Syzygium aromaticum and Zanthoxylum xanthoxyloides | [55] |
| Pythium spp. | Root rot | Thymus spp. | [90] | |
| Mikania scandens | [57] | |||
| Essential Oil Distilled from | Target Bacteria and Caused Disease | References | ||
|---|---|---|---|---|
| Achillea biebersteinii | Gram positive bacteria | Clavibacter michiganensis | Ring rot disease | [119] |
| Achillea millefolium | ||||
| Ocimum ciliatum | Rhodococcus fascians | Leafy gall disease | [116] | |
| Origanum heracleoticum | Clavibacter michiganensis | Ring rot disease | [88] | |
| Origanum majorana | ||||
| Origanum onites | [116] | |||
| Salmea scandens | [99] | |||
| Satureja hortensis | [120] | |||
| Satureja spicigera | [119] | |||
| Solidago canadensis L. | [82] | |||
| Tanacetum aucheranum | [117] | |||
| Thymus fallax | [119] | |||
| Achillea biebersteinii | Gram negative bacteria | Erwinia spp. | [119] | |
| Pseudomonas spp. | Bacterial canker | |||
| Xanthomonas spp. | Bacterial spots and blights | |||
| Achillea millefolium | Erwinia spp. | |||
| Pseudomonas spp. | Bacterial canker | |||
| Xanthomonas spp. | Bacterial spots and blights | |||
| Citrus aurantium L. | Agrobacterium tumefaciens | Crown gall | [121] | |
| Dickeya solani | Black leg and soft rot | |||
| Erwinia amylovora | Fire blight | |||
| Citrus reticulata | Pseudomonas aeruginosa | Soft rot | [122] | |
| Cleistocalyx operculatus | Xanthomonas spp. | Bacterial spots and blights | [123] | |
| Cynara scolymus (stems) | Gram negative bacteria | Erwinia amylovora | Fire blight | [124] |
| Erwinia carotovora | Soft rot | |||
| Pseudomonas syringae | Bacterial canker | |||
| Xanthomonas vesicatoria | Bacterial leaf spot | |||
| Eriocephalus africanus L. | Agrobacterium tumefaciens | Crown gall | [125] | |
| Dickeya solani | Black leg and soft rot | |||
| Erwinia amylovora | Fire blight | |||
| Pseudomonas cichorii | Leaf blight and spots | |||
| Serratia pulmithica | ||||
| Juglans regia L. (shells) | Erwinia amylovora | Fire blight | [124] | |
| Erwinia carotovora | Soft rot | |||
| Pseudomonas syringae | Bacterial canker | |||
| Xanthomonas vesicatoria | Bacterial leaf spot | |||
| Metasequoia glyptostroboides | Xanthomonas spp. | Bacterial spots and blights | [89] | |
| Ocimum ciliatum | Agrobacterium vitis | Crown gall | [116] | |
| Brenneria nigrifluens | Cankers | |||
| Pantoea stewartii | Stewart’s wilt and leaf blight | |||
| Pseudomonas spp. | Bacterial canker | |||
| Ralstonia solanacearum | Bacterial wilt | |||
| Xanthomonas spp. | Bacterial spots and blights | [123] | ||
| Ocimum basilicum | Pseudomonas aeruginosa | Soft rot | [76] | |
| Origanum heracleoticum | Pseudomonas spp. | Bacterial canker | [88] | |
| Xanthomonas sp. | Bacterial spots and blights | |||
| Origanum majorana | Pseudomonas spp. | Bacterial canker | ||
| Xanthomonas sp. | Bacterial spots and blights | |||
| Origanum onites | Erwinia spp. | [116] | ||
| Pseudomonas spp. | Bacterial canker | |||
| Xanthomonas spp. | Bacterial spots and blights | |||
| Origanum vulgare | Erwinia amylovora | Fire blight | [124] | |
| Erwinia carotovora | Soft rot | |||
| Pseudomonas syringae | Bacterial canker | |||
| Xanthomonas vesicatoria | Bacterial leaf spot | |||
| Pseudomonas syringae | Bacterial canker | [126] | ||
| Pseudomonas spp. | Bacterial canker | [127] | ||
| Piper sarmentosum | Xanthomonas oryzae pv. oryzae | Bacterial blight | [108] | |
| Xanthomonas oryzae pv. oryzicola | Bacterial blight | |||
| Salmea scandens | Pseudomonas syringae | Bacterial canker | [99] | |
| Erwinia carotovora | Soft rot | |||
| Erwinia spp. | ||||
| Pseudomonas spp. | Bacterial canker | |||
| Salmea scandens | Xanthomonas spp. | Bacterial spots and blights | [99] | |
| Erwinia carotovora | Soft rot | |||
| Satureja hortensis | Erwinia spp. | [120] | ||
| Pseudomonas spp. | Bacterial spots and blights | |||
| Xanthomonas spp. | Bacterial spots and blights | |||
| Satureja spicigera | Erwinia spp. | [118] | ||
| Pseudomonas spp. | Bacterial canker | |||
| Xanthomonas spp. | Bacterial spots and blights | |||
| Solidago canadensis L. | Pseudomonas spp. | Bacterial canker | [82] | |
| Xanthomonas sp. | Bacterial spots and blights | |||
| Syzygium aromaticum | Gram negative bacteria | Erwinia amylovora | Fire blight | [124] |
| Erwinia carotovora | Soft rot | |||
| Pseudomonas syringae | Bacterial canker | |||
| Xanthomonas vesicatoria | Bacterial leaf spot | |||
| Tanacetum aucheranum | Agrobacterium tumefaciens | Crown gall | [117] | |
| Erwinia spp. | ||||
| Pseudomonas spp. | Bacterial canker | |||
| Xanthomonas spp. | Bacterial spots and blights | |||
| Tanacetum chiliophyllum | Agrobacterium tumefaciens | Crown gall | [118] | |
| Erwinia spp. | ||||
| Pseudomonas spp. | Bacterial canker | |||
| Xanthomonas spp. | Bacterial spots and blights | |||
| Thymus fallax | Erwinia spp. | [126] | ||
| Pseudomonas spp. | Bacterial canker | |||
| Xanthomonas spp. | Bacterial spots and blights | |||
| Thymus vulgaris | Pseudomonas syringae | Bacterial canker | [127] | |
| Pseudomonas spp. | Bacterial canker | |||
| Vetiveria zizanioides | Pseudomonas aeruginosa | Soft rot | [76] | |
| Zataria multiflora | Xanthomonas campestris | Black rot and leaf spot | [128] | |
| 11 different plants | Erwinia amylovora | Fire blight | [129] | |
| 18 egyptian plants | Agrobacterium tumefaciens | Crown gall | [59] | |
| Erwinia carotovora | Soft rot | |||
| Essential Oil Distilled from | Plant Tested | References |
|---|---|---|
| Achillea gypsicola | Amaranthus retroflexus | [137] |
| Chenopodium album | ||
| Cirsium arvense | ||
| Lactuca serriola | ||
| Rumex crispus | ||
| Achillea biebersteinii | Amaranthus retroflexus | [137] |
| Chenopodium album | ||
| Cirsium arvense | ||
| Lactuca serriola | ||
| Rumex crispus | ||
| Angelica glauca | Lemna minor | [73] |
| Citrus x limon L. | Portulaca oleracea | [140] |
| Citrus aurantiifolia | Avena fatua | [131] |
| Echinochloa crus-galli | ||
| Phalaris minor | ||
| Coriandrum sativum L. | Amaranthus retroflexus | [141] |
| Chenopodium album | ||
| Echinochloa crus-galli | ||
| Eucalyptus spp. | Annual ryegrass | [142] |
| Echinochloa crus-galli | [143] | |
| Lolium multiflorum | ||
| Nicotiana glauca | ||
| Phalaris minor | [139] | |
| Parthenium hysterophorus | [138] | |
| Portulaca oleracea | [143] | |
| Sinapis arvensis | [84] | |
| Phalaris canariensis | [36] | |
| Solanum elaeagnifolium | ||
| Lavandula spp. | Lolium rigidum | [144] |
| 12 Mediterranean species | Lactuca sativa | [136] |
| Lepidium sativum | ||
| Raphanus sativus | ||
| Origanum acutidens | Amaranthus retroflexus | [135] |
| Rumex crispus | ||
| Chenopodium album | ||
| Origanum vulgare L. | Hordeum vulgare | [145] |
| Lepidium sativum | ||
| Matricaria chamomilla L. | [146] | |
| Sinapsis alba | [145] | |
| Triticum aestivum | ||
| Peumus boldus | Portulaca oleracea | [140] |
| Pinus nigra | Phalaris canariensis | [147] |
| Trifolium campestre | ||
| Sinapis arvensis | ||
| Pinus pinea | Sinapis arvensis | [25] |
| Raphanus raphanistrum | ||
| Lolium rigidum | ||
| Plectranthus rugosus | Lemna minor | [73] |
| Rosmarinus officinalis | Amaranthus retroflexus | [130] |
| Matricaria chamomilla L. | [146] | |
| Phalaris minor | [100] | |
| Rhaphanus sativus | [130] | |
| Silybum marianum | [100] | |
| Trifolium incarnatum | ||
| Syzygium aromaticum | Common lambsquarters | [148] |
| Redwood pigweed | ||
| Tagetes erecta | Echinochloa crus-galli L. Beauv. | [149] |
| Tanacetum aucheranum | Amaranthus retroflexus | [117] |
| Chenopodium album | ||
| Rumex crispus | ||
| Tanacetum chiliophyllum | Amaranthus retroflexus | |
| Chenopodium album | ||
| Rumex crispus | ||
| Tetraclinis articulata | Sinapis arvensis | [89] |
| Phalaris canariensis | ||
| 25 various plants | Taraxacum officinale | [150] |
| Valeriana wallichii | Lemna minor | [73] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods 2020, 9, 365. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9030365
Raveau R, Fontaine J, Lounès-Hadj Sahraoui A. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods. 2020; 9(3):365. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9030365
Chicago/Turabian StyleRaveau, Robin, Joël Fontaine, and Anissa Lounès-Hadj Sahraoui. 2020. "Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review" Foods 9, no. 3: 365. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9030365