A Review of Coffee By-Products Including Leaf, Flower, Cherry, Husk, Silver Skin, and Spent Grounds as Novel Foods within the European Union
Abstract
:1. Introduction
2. Materials and Methods
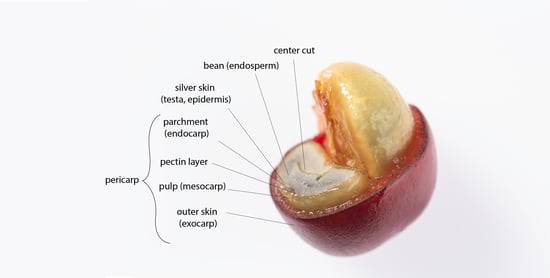
3. Coffee By-Products
3.1. Flowers
3.2. Leaves
3.3. Stems, Twigs, Wood
3.4. Cherry Pulp
3.5. Parchment
3.6. Cherry Husk
3.7. Green Coffee
3.8. Silver Skin
3.9. Spent Coffee Grounds
4. Novel Food Status of Coffee By-Products
4.1. Evidence for Human Consumption of Coffee By-Products within the EU
4.2. The Way Forward: Novel Food Approval of Coffee By-Products
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lachenmeier, D.W.; Teipel, J.; Scharinger, A.; Kuballa, T.; Walch, S.G.; Grosch, F.; Bunzel, M.; Okaru, A.O.; Schwarz, S. Fully automated identification of coffee species and simultaneous quantification of furfuryl alcohol using NMR spectroscopy. J. AOAC Int. 2020, 103, 306–314. [Google Scholar] [CrossRef]
- International Coffee Organization. Trade Statistics Tables—Production. Available online: http://www.ico.org/prices/po-production.pdf (accessed on 7 April 2020).
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- De Melo Pereira, G.V.; de Carvalho Neto, D.P.; Magalhães Júnior, A.I.; do Prado, F.G.; Pagnoncelli, M.G.B.; Karp, S.G.; Soccol, C.R. Chemical composition and health properties of coffee and coffee by-products. Adv. Food Nutr. Res. 2020, 91, 65–96. [Google Scholar] [CrossRef]
- European Union. European Union Regulation (EU) 2015/2283 of the European parliament and of the council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. Off. J. Eur. Union 2015, L327, 1–22. [Google Scholar]
- European Union. European Union Commission implementing regulation (EU) 2017/2468 of 20 December 2017 laying down administrative and scientific requirements concerning traditional foods from third countries in accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. Off. J. Eur. Union 2017, L351, 55–63. [Google Scholar]
- European Union. European Union Commission implementing regulation (EU) 2017/2469 of 20 December 2017laying down administrative and scientific requirements for applications referred to in Article 10 of Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. Off. J. Eur. Union 2017, L351, 64–71. [Google Scholar]
- European Union. European Union Commission implementing regulation (EU 2017/2470 of 20 December 2017 establishing the Union list of novel foods in accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. Off. J. Eur. Union 2017, L351, 72–201. [Google Scholar]
- EFSA. Novel Food. Available online: http://www.efsa.europa.eu/en/topics/topic/novel-food (accessed on 3 May 2020).
- European Union. Coffea sp. Available online: https://ec.europa.eu/food/safety/novel_food/catalogue/search/public/index.cfm?ascii=Coffea (accessed on 20 March 2020).
- Murthy, P.S.; Naidu, M.M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Clifford, M.N.; Williams, T.; Bridson, D. Chlorogenic acids and caffeine as possible taxonomic criteria in Coffea and Psilanthus. Phytochemistry 1989, 28, 829–838. [Google Scholar] [CrossRef]
- Davis, A.P.; Govaerts, R.; Bridson, D.M.; Stoffelen, P. An annotated taxonomic conspectus of the genus Coffea (Rubiaceae). Bot. J. Linn. Soc. 2006, 152, 465–512. [Google Scholar] [CrossRef] [Green Version]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Lehrbuch der Lebensmittelchemie, 6th ed.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Narita, Y.; Inouye, K. Review on utilization and composition of coffee silverskin. Food Res. Int. 2014, 61, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.M.T.; Cho, E.J.; Song, Y.; Oh, C.H.; Funada, R.; Bae, H.-J. Use of coffee flower as a novel resource for the production of bioactive compounds, melanoidins, and bio-sugars. Food Chem. 2019, 299, 125120. [Google Scholar] [CrossRef]
- Ratanamarno, S.; Surbkar, S. Caffeine and catechins in fresh coffee leaf (Coffea arabica) and coffee leaf tea. Maejo Int. J. Sci. Technol. 2017, 11, 211–218. [Google Scholar]
- Campa, C.; Petitvallet, A. Beneficial compounds from coffee leaves. In Achieving Sustainable Cultivation of Coffee; Lashermes, P., Ed.; Burleigh Dodds Science Publishing Limited: Milton, UK, 2018; pp. 237–258. [Google Scholar]
- Kamiya, H. Manufacturing method of beverage raw material. Patent No. JP-2017153463-A, 1 March 2016. [Google Scholar]
- Rilma, N.; Anwar, K.; Tuty, A.; Deddi, P.P. Kahwa daun: Traditional knowledge of a coffee leaf herbal tea from West Sumatera, Indonesia. J. Ethn. Foods 2018, 5, 286–291. [Google Scholar] [CrossRef]
- Yuwono, S.S.; Fibrianto, K.; Wahibah, L.Y.; Wardhana, A.R. Sensory attributes profiling of dampit robusta coffee leaf tea (Coffea canephora). Carpathian J. Food Sci. Technol. 2019, 164–175. [Google Scholar] [CrossRef]
- Madahava Naidu, M.; Vijayanada, P.; Usha Devi, A.; Vijayalakshmi, M.R.; Ramalakshmi, K. Utilization of coffee by-products in food industry, preparation of jam using coffee pulp as raw material. In Plantation Crops Research and Development in the New Millennium: PLACROSYM XIV; Rethinam, P., Ed.; Indian Society for Plantation Crops: Kasaragod, India, 2004; pp. 201–203. [Google Scholar]
- Ramirez Velez, A.; Jaramillo Lopez, J.C. Process for Obtaining Honey and/or Flour of Coffee from the Pulp or Husk and the Mucilage of the Coffee Bean. U.S. Patent No. US20150017270A1, 14 December 2011. [Google Scholar]
- Torres-Valenzuela, L.S.; Serna-Jiménez, J.A.; Martínez, K. Coffee by-products: Nowadays and perspectives. IntechOpen 2019. [Google Scholar] [CrossRef]
- Heeger, A.; Kosińska-Cagnazzo, A.; Cantergiani, E.; Andlauer, W. Bioactives of coffee cherry pulp and its utilisation for production of Cascara beverage. Food Chem. 2017, 221, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Brenna, C. What Is Cascara? Available online: https://www.freshcup.com/what-is-cascara/ (accessed on 16 March 2020).
- Velissariou, M.; Laudano, R.J.; Edwards, P.M.; Stimpson, S.M.; Jeffries, R.L. Beverage derived from the extract of coffee cherry husks and coffee cherry pulp. U.S. Patent No. US7833560B2, 18 March 2005. [Google Scholar]
- Carlsen, Z. Magic in the Moonshine: Cascara Booze Is Here. Available online: https://sprudge.com/magic-in-the-moonshine-cascara-booze-is-here-115811.html (accessed on 16 March 2020).
- Discarded Spirits Co. Discarded Vermouth. Available online: https://www.discardedspirits.com/products/discarded-vermouth (accessed on 16 March 2020).
- Ota, K. Coffee as a global beverage before 1700. J. Int. Econ. Stud. 2018, 3, 43–55. [Google Scholar] [CrossRef]
- Maxwell, G.W. Poor man’s coffee? Yemeni qishr, qahwa, and qat. Tea Coffee Trade J. 1996, 168, 64–68. [Google Scholar]
- Iriondo-DeHond, A.; Aparicio García, N.; Fernandez-Gomez, B.; Guisantes-Batan, E.; Velázquez Escobar, F.; Blanch, G.P.; San Andres, M.I.; Sanchez-Fortun, S.; del Castillo, M.D. Validation of coffee by-products as novel food ingredients. Innovative Food Sci. Emerging Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Onakpoya, I.; Terry, R.; Ernst, E. The use of green coffee extract as a weight loss supplement: A systematic review and meta-analysis of randomised clinical trials. Gastroent. Res. Pract. 2011, 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J. Nutr. 2008, 138, 2309–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcason, W. What is green coffee extract? J. Acad. Nutr. Diet. 2013, 113, 364. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.J. The Technology of converting green coffee into the beverage. In Coffee: Botany, Biochemistry and Production of Beans and Beverage; Clifford, M.N., Willson, K.C., Eds.; Springer: Boston, MA, USA, 1985; pp. 375–393. [Google Scholar]
- Macheiner, L.; Schmidt, A.; Schreiner, M.; Mayer, H.K. Green coffee infusion as a source of caffeine and chlorogenic acid. J. Food Compos. Anal. 2019, 84, 103307. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Production and application of xylanase from Penicillium sp. utilizing coffee by-products. Food Bioprocess. Technol. 2010, 5, 657–664. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Recovery of Phenolic Antioxidants and functional compounds from coffee industry by-products. Food Bioprocess. Technol. 2010, 5, 897–903. [Google Scholar] [CrossRef]
- Costa, A.S.G.; Alves, R.C.; Vinha, A.F.; Costa, E.; Costa, C.S.G.; Nunes, M.A.; Almeida, A.A.; Santos-Silva, A.; Oliveira, M.B.P.P. Nutritional, chemical and antioxidant/pro-oxidant profiles of silverskin, a coffee roasting by-product. Food Chem. 2018, 267, 28–35. [Google Scholar] [CrossRef]
- Borrelli, R.C.; Esposito, F.; Napolitano, A.; Ritieni, A.; Fogliano, V. Characterization of a new potential functional ingredient: Coffee silverskin. J. Agric. Food Chem. 2004, 52, 1338–1343. [Google Scholar] [CrossRef]
- Jiménez-Zamora, A.; Pastoriza, S.; Rufián-Henares, J.A. Revalorization of coffee by-products. Prebiotic, antimicrobial and antioxidant properties. LWT Food Sci. Technol. 2015, 61, 12–18. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess. Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef] [Green Version]
- Pourfarzad, A.; Mahdavian-Mehr, H.; Sedaghat, N. Coffee silverskin as a source of dietary fiber in bread-making: Optimization of chemical treatment using response surface methodology. LWT Food Sci. Technol. 2013, 50, 599–606. [Google Scholar] [CrossRef]
- Garcia-Serna, E.; Martinez-Saez, N.; Mesias, M.; Morales, F.; Castillo, M. Use of coffee silverskin and Stevia to improve the formulation of biscuits. Pol. J. Food Nutr. Sci. 2014, 64, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Ateş, G.; Elmacı, Y. Coffee silverskin as fat replacer in cake formulations and its effect on physical, chemical and sensory attributes of cakes. LWT Food Sci. Technol. 2018, 90, 519–525. [Google Scholar] [CrossRef]
- Ateş, G.; Elmacı, Y. Physical, chemical and sensory characteristics of fiber-enriched cakes prepared with coffee silverskin as wheat flour substitution. Food Measure 2019, 13, 755–763. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; del Castillo, M.D. Development of sustainable novel foods and beverages based on coffee by-products for chronic diseases. In Encyclopedia of Food Security and Sustainability; Ferranti, P., Berry, E., Jock, A., Eds.; Elsevier: San Diego, CA, USA, 2018; pp. 307–315. [Google Scholar]
- Martinez-Saez, N.; Ullate, M.; Martin-Cabrejas, M.A.; Martorell, P.; Genovés, S.; Ramon, D.; del Castillo, M.D. A novel antioxidant beverage for body weight control based on coffee silverskin. Food Chem. 2014, 150, 227–234. [Google Scholar] [CrossRef]
- Sampaio, A.; Dragone, G.; Vilanova, M.; Oliveira, J.M.; Teixeira, J.A.; Mussatto, S.I. Production, chemical characterization, and sensory profile of a novel spirit elaborated from spent coffee ground. LWT Food Sci. Technol. 2013, 54, 557–563. [Google Scholar] [CrossRef] [Green Version]
- del Castillo, M.D.; Ibanez, M.E.; Amigo-Benavent, M.; Herrero, M.; Plaza, M.; Ullate, M. Aplicacion de Pruductos de la Cascarilla del Cafe en Cosmetica Antienvejecimiento y Alimentacion Funcional. Patent No. WO2013004873A1, 4 July 2011. [Google Scholar]
- Martinez-Saez, N.; García, A.T.; Pérez, I.D.; Rebollo-Hernanz, M.; Mesías, M.; Morales, F.J.; Martín-Cabrejas, M.A.; Del Castillo, M.D. Use of spent coffee grounds as food ingredient in bakery products. Food Chem. 2017, 216, 114–122. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, K.; Martinez-Saez, N.; Rebollo-Hernanz, M.; Del Castillo, M.D.; Gaytán-Martínez, M.; Campos-Vega, R. In vitro health promoting properties of antioxidant dietary fiber extracted from spent coffee (Coffee arabica L.) grounds. Food Chem. 2018, 261, 253–259. [Google Scholar] [CrossRef]
- Campos-Vega, R. Proseco de obtencion de fibra dietaria antioxidante natural de subproductos mediante calentamiento ohmico y compuesto alto en fibra dietaria antioxidante natural de cafe usado. Patent No. MX/a/2016008578, 2016. [Google Scholar]
- Campos-Vega, R.; Vázquez-Sánchez, K.; Martinez-Saez, N.; Castillo, M. Antioxidant coffee dietary fiber for gastrointestinal health and diabetes. In Proceedings of the 20th International Conference of FFC and 8th International Symposium of Academic Society of Functional Foods and Bioactive Compounds, Boston, MA, USA, 22–23 September 2016. [Google Scholar]
- Kaffe Bueno ApS. Coffee Flour (Defatted Coffee Arabica Seed Powder). Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/novel-food_sum_ongoing-app_2018-0698.pdf (accessed on 16 March 2020).
- Mirón-Mérida, V.A.; Yáñez-Fernández, J.; Montañez-Barragán, B.; Barragán Huerta, B.E. Valorization of coffee parchment waste (Coffea arabica) as a source of caffeine and phenolic compounds in antifungal gellan gum films. LWT Food Sci. Technol. 2019, 101, 167–174. [Google Scholar] [CrossRef]
- Mayer, J.L.S.; Carmello-Guerreiro, S.M.; Mazzafera, P. A functional role for the colleters of coffee flowers. AoB Plants 2013, 5. [Google Scholar] [CrossRef]
- Hong, N. Today, We’re Drinking Flowers. Available online: https://www.drinkmagazine.asia/2017/06/16/today-drinking-flowers/ (accessed on 16 March 2020).
- Wintgens, J.N. The coffee plant. In Coffee: Growing, Processing, Sustainable Production: A Guidebook for Growers, Processors, Traders and Researchers; Wintgens, J.N., Ed.; Wiley-VCH: Weinheim, Germany, 2008; pp. 1–24. [Google Scholar]
- Chen, X. A review on coffee leaves: Phytochemicals, bioactivities and applications. Crit. Rev. Food Sci. Nutr. 2019, 59, 1008–1025. [Google Scholar] [CrossRef] [PubMed]
- Ross, I.A. Medicinal plants of the world, volume 3. In Chemical Constituents, Traditional and Modern Medicinal Uses; Humana Press Inc.: Totowa, NJ, USA, 2005. [Google Scholar]
- Chen, X.-M.; Ma, Z.; Kitts, D.D. Effects of processing method and age of leaves on phytochemical profiles and bioactivity of coffee leaves. Food Chem. 2018, 249, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Patay, É.B.; Bencsik, T.; Papp, N. Phytochemical overview and medicinal importance of Coffea species from the past until now. Asian Pac. J. Trop. Med. 2016, 9, 1127–1135. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Ling, X.; Bai, X.; Guo, T.; Li, J. Method for Making Granular Coffee Leaf Tea. Patent No. CN-105192194-A, 17 June 2014. [Google Scholar]
- Shan, Y. Making Method for Coffee Leave Tea. Patent No. CN-104719522-A, 9 April 2015. [Google Scholar]
- Ito, K. Preparation of Tea Extract. Patent No. JP-3115464-B2, 16 December 1993. [Google Scholar]
- Inoue, M. Coffee Leaf Tea and Its Preparation. Patent No. JP-H08173111-A, 22 December 1994. [Google Scholar]
- Tagagaki, K.; Tsuzaki, S. Healthy Tea and Healthy Drink and Method of Manufacturing the Same. Patent No. JP-2002065227-A, 31 August 2000. [Google Scholar]
- Iwai, K.; Nakabayashi, Y. Coffee Leave Tea and Method for Producing the Same. Patent No. JP-2002191332-A, 27 December 2000. [Google Scholar]
- Hirose, Y.; Yoshimura, K.; Yamamoto, K. Manufacturing Method of Coffee Leave Tea Raw Material, and Coffee Leave Tea Beverage Using the Same. Patent No. JP-2013106536-A, 18 November 2011. [Google Scholar]
- Campa, C.; Mondolot, L.; Rakotondravao, A.; Bidel, L.P.R.; Gargadennec, A.; Couturon, E.; La Fisca, P.; Rakotomalala, J.-J.; Jay-Allemand, C.; Davis, A.P. A survey of mangiferin and hydroxycinnamic acid ester accumulation in coffee (Coffea) leaves: Biological implications and uses. Ann. Bot. 2012, 110, 595–613. [Google Scholar] [CrossRef] [Green Version]
- Jyotshna; Khare, P.; Shanker, K. Mangiferin: A review of sources and interventions for biological activities. BioFactors 2016, 42, 504–514. [Google Scholar] [CrossRef]
- Luczkiewicz, P.; Kokotkiewicz, A.; Dampc, A.; Luczkiewicz, M. Mangiferin: A promising therapeutic agent for rheumatoid arthritis treatment. Med. Hypotheses 2014, 83, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.; Mohan Rao, L.J. An outlook on chlorogenic acids-occurrence, chemistry, technology, and biological activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Ashihara, H.; Monteiro, A.M.; Gillies, F.M.; Crozier, A. Biosynthesis of caffeine in leaves of coffee. Plant Phys. 1996, 111, 747–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S. Vehicle Perfume Useful for Preventing Sleep Comprises Lavender, Pelargonium Odoratissimum, Plum Blossom, Lily, Michelia, Laggera Pterodonta, Coffee Leaf, Ethanol, Plant Extracts, Citric Acid, Folic Acid and Sodium Chloride. Patent No. CN-107998268-A, 4 December 2017. [Google Scholar]
- Madya, A.P. Sutriyo Gel Composition Used for Manufacturing Gel-Shaped Product as Coagulant of Dead Skin Cells Protein, Contains Coffee Leaf Powder, Triethanolamine, Carbomer 940, Sodium Laureth Sulfate, Cocamidopropyl Betaine and Methyl Paraben. Patent No. ID-201606611-A, 6 May 2015. [Google Scholar]
- Teruel, E.B. Novel tobacco substitute. Patent No. US 2008/0017208 A1, 20 July 2006. [Google Scholar]
- Xu, H. Cigarette with Coffee Flavor and Its Preparing Method. Patent No. CN-1206948-C, 15 January 2003. [Google Scholar]
- Ye, H. Feed for Promoting Growth and Development of Broilers and Preparation Method Thereof. Patent No. CN-104431535-A, 10 December 2014. [Google Scholar]
- Furukawa, Y.; Hizaki, S.; Nii, H.; Yamauchi, M.; Yoshimur, H. Lactic Acid Proliferating Agent and Its Production. Patent No. JP-H06125771-A, 21 October 1992. [Google Scholar]
- Hizaki, S.; Yamauchi, M. Enteric-Coated Lactobacillus Granule. Patent No. JP-H06133735-A, 23 October 1992. [Google Scholar]
- Ragot, P.; Barat, L.; Rousseau, C.; Pons, E. Reconstituted Plant Material and Its Use for Packaging, Wrapping and Food Appliances. Patent No. US-20170174404A1, 28 March 2014. [Google Scholar]
- Kim, T.H.; Lee, S.S.; Lee, H.H.; Bae, H.K.; Song, J.A.; Kim, J.M. Absorbent Pad Containing Coffee Leaves. Patent No. KR-101754481-B1, 31 August 2015. [Google Scholar]
- Ucc Ueshima Kohi, K. Deodoriser for Hospital Beds-Contains Adsorption Agent e.g., Dried Coffee Leaves or Tea Waste. Patent No. JP-10314286-A, 15 May 1997. [Google Scholar]
- EFSA. Technical Report on the Notification of Infusion from Coffee Leaves (Coffea Arabica L. and/or Coffea Canephora Pierre ex A. Froehner) as a Traditional Food from a Third Country Pursuant to Article 14 of Regulation (EU) 2015/2283; EFSA Supporting Publications; EFSA: Parma, Italy, 2020; Volume 17, EN-1783. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Scientific opinion on the safety of green tea catechins. EFSA J. 2018, 16, 5239. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults—Results of a systematic review. Regul. Toxicol. Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef]
- Prado, Y.; Merino, N.; Acosta, J.; Herrera, J.A.; Luque, Y.; Hernández, I.; Prado, E.; Garrido, G.; Delgado, R.; Rodeiro, I. Acute and 28-day subchronic toxicity studies of mangiferin, a glucosyl xanthone isolated from Mangifera indica L. stem bark. J. Pharm. Pharmacogn. Res. 2015, 3, 13–23. [Google Scholar]
- Reddeman, R.A.; Glávits, R.; Endres, J.R.; Clewell, A.E.; Hirka, G.; Vértesi, A.; Béres, E.; Szakonyiné, I.P. A Toxicological evaluation of mango leaf extract (Mangifera indica) containing 60% mangiferin. J. Toxicol. 2019, 2019, 4763015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Trade Centre. The Coffee Guide—Grading and Classification. Available online: http://www.thecoffeeguide.org/coffee-guide/world-coffee-trade/grading-and-classification/ (accessed on 25 March 2020).
- Bundesministerium für Verbraucherschutz, Ernährung und Landwirtschaft. Verordnung über Kaffee, Kaffee- und Zichorien-Extrakte vom 15. November 2001 (BGBl. I S. 3107), die zuletzt durch Artikel 6 der Verordnung vom 5. Juli 2017 (BGBl. I S. 2272) geändert worden ist: KaffeeV. Bundesgesetzblatt I 2017, G5702, 2272–2289. [Google Scholar]
- Bonilla-Hermosa, V.A.; Duarte, W.F.; Schwan, R.F. Utilization of coffee by-products obtained from semi-washed process for production of value-added compounds. Bioresour. Technol. 2014, 166, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Franca, A.S.; Oliveira, L.S. Coffee processing solid wastes: Current uses and future perspectives (chapter 8). In Agricultural Wastes; Ashworth, G.S., Azevedo, P., Eds.; Nova Science: New York, NY, USA, 2009. [Google Scholar]
- Clifford, M.N.; Ramirez-Martinez, J.R. Phenols and caffeine in wet-processed coffee beans and coffee pulp. Food Chem. 1991, 40, 35–42. [Google Scholar] [CrossRef]
- Benitez, V.; Rebollo-Hernanz, M.; Hernanz, S.; Chantres, S.; Aguilera, Y.; Martin-Cabrejas, M.A. Coffee parchment as a new dietary fiber ingredient: Functional and physiological characterization. Food Res. Int. 2019, 122, 105–113. [Google Scholar] [CrossRef]
- Bekalo, S.A.; Reinhardt, H.-W. Fibers of coffee husk and hulls for the production of particleboard. Mater. Struct. 2010, 43, 1049–1060. [Google Scholar] [CrossRef]
- Giuliano, J. What Is Cascara?—Exploring Coffee Cherry Tea. Available online: http://www.manualcoffeebrewing.com/what-is-cascara-how-to-brew-is-and-where-to-get-it-exploring-coffee-cherry-tea/ (accessed on 19 March 2020).
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; Gonzalez de Mejia, E. Phenolic compounds from coffee by-products modulate adipogenesis-related inflammation, mitochondrial dysfunction, and insulin resistance in adipocytes, via insulin/PI3K/AKT signaling pathways. Food Chem. Toxicol. 2019, 132, 110672. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; Gonzalez de Mejia, E. Relationship of the phytochemicals from coffee and cocoa by-products with their potential to modulate biomarkers of metabolic syndrome in vitro. Antioxidants 2019, 8, 279. [Google Scholar] [CrossRef] [Green Version]
- Gouvea, B.M.; Torres, C.; Franca, A.S.; Oliveira, L.S.; Oliveira, E.S. Feasibility of ethanol production from coffee husks. Biotechnol. Lett. 2009, 31, 1315–1319. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Lima, N.; Taniwaki, M.H. Coffee, mycotoxins and climate change. Food Res. Int. 2014, 61, 1–15. [Google Scholar] [CrossRef]
- EFSA. Opinion of the scientific panel on contaminants in the food chain [CONTAM] related to ochratoxin A in food. EFSA J. 2006, 4, 365. [Google Scholar] [CrossRef]
- Joët, T.; Laffargue, A.; Descroix, F.; Doulbeau, S.; Bertrand, B.; de kochko, A.; Dussert, S. Influence of environmental factors, wet processing and their interactions on the biochemical composition of green Arabica coffee beans. Food Chem. 2010, 118, 693–701. [Google Scholar] [CrossRef]
- González, A.G.; Pablos, F.; Martίn, M.J.; León-Camacho, M.; Valdenebro, M.S. HPLC analysis of tocopherols and triglycerides in coffee and their use as authentication parameters. Food Chem. 2001, 73, 93–101. [Google Scholar] [CrossRef]
- Naidu, M.; Sulochanamma, G.; Sampathu, S.R.; Srinivas, P. Studies on extraction and antioxidant potential of green coffee. Food Chem. 2008, 107, 377–384. [Google Scholar] [CrossRef]
- Campa, C.; Doulbeau, S.; Dussert, S.; Hamon, S.; Noirot, M. Qualitative relationship between caffeine and chlorogenic acid contents among wild species. Food Chem. 2005, 93, 135–139. [Google Scholar] [CrossRef]
- Ky, C.-L.; Louarn, J.; Dussert, S.; Guyot, B.; Hamon, S.; Noirot, M. Caffeine, trigonelline, chlorogenic acids and sucrose diversity in wild Coffea arabica L. and C. canephora P. accessions. Food Chem. 2001, 75, 223–230. [Google Scholar] [CrossRef]
- Anthony, F.; Clifford, M.N.; Noirot, M. Biochemical diversity in the genus Coffea L.: Chlorogenic acids, caffeine and mozambioside contents. Genet. Resour. Crop. Evol. 1993, 40, 61–70. [Google Scholar] [CrossRef]
- Clifford, M.N. Chemical and physical aspects of green coffee and coffee products. In Coffee: Botany, Biochemistry and Production of Beans and Beverage; Clifford, M.N., Willson, K.C., Eds.; Springer: Boston, MA, USA, 1985; pp. 305–374. [Google Scholar]
- Arya, M.; Rao, L.J.M. An impression of coffee carbohydrates. Crit. Rev. Food Sci. Nutr. 2007, 47, 51–67. [Google Scholar] [CrossRef]
- Oestreich-Janzen, S. Chemistry of coffee. In Comprehensive Natural Products II: Chemistry and Biology; Mander, L., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1085–1117. [Google Scholar]
- Mazzafera, P.; Silvarolla, M.B. Caffeine content variation in single green Arabica coffee seeds. Seed Sci. Res. 2010, 20, 163–167. [Google Scholar] [CrossRef]
- Dessalegn, Y.; Labuschagne, M.T.; Osthoff, G.; Herselman, L. Genetic diversity and correlation of bean caffeine content with cup quality and green bean physical characteristics in coffee (Coffea arabica L.). J. Sci. Food Agric. 2008, 88, 1726–1730. [Google Scholar] [CrossRef]
- Lang, R.; Yagar, E.F.; Eggers, R.; Hofmann, T. Quantitative investigation of trigonelline, nicotinic acid, and nicotinamide in foods, urine, and plasma by means of LC-MS/MS and stable isotope dilution analysis. J. Agric. Food Chem. 2008, 56, 11114–11121. [Google Scholar] [CrossRef] [PubMed]
- Stadler, R.H.; Varga, N.; Milo, C.; Schilter, B.; Vera, F.A.; Welti, D.H. Alkylpyridiniums. 2. Isolation and quantification in roasted and ground coffees. J. Agric. Food Chem. 2002, 50, 1200–1206. [Google Scholar] [CrossRef]
- Stadler, R.H.; Varga, N.; Hau, J.; Vera, F.A.; Welti, D.H. Alkylpyridiniums. 1. Formation in model systems via thermal degradation of trigonelline. J. Agric. Food Chem. 2002, 50, 1192–1199. [Google Scholar] [CrossRef]
- Suzuki, A.; Kagawa, D.; Ochiai, R.; Tokimitsu, I.; Saito, I. Green coffee bean extract and its metabolites have a hypotensive effect in spontaneously hypertensive rats. Hypertens. Res. 2002, 25, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Thom, E. The effect of chlorogenic acid enriched coffee on glucose absorption in healthy volunteers and its effect on body mass when used long-term in overweight and obese people. J. Int. Med. Res. 2007, 35, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Arai, Y.; Mitsui, Y.; Kusaura, T.; Okawa, W.; Kajihara, Y.; Saito, I. The blood pressure-lowering effect and safety of chlorogenic acid from green coffee bean extract in essential hypertension. Clin. Exp. Hypertens. 2006, 28, 439–449. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, N.A.; Sandini, T.M.; Cornelio-Santiago, H.P.; Martinelli, E.C.L.; Raspantini, L.E.R.; Raspantini, P.C.; Momo, C.; de Oliveira, A.L.; Fukumasu, H. Acute and subacute (28 days) toxicity of green coffee oil enriched with diterpenes cafestol and kahweol in rats. Regul. Toxicol. Pharmacol. 2020, 110, 104517. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific opinion on the safety of caffeine. EFSA J. 2015, 13, 4102. [Google Scholar] [CrossRef] [Green Version]
- Vaclavik, L.; Vaclavikova, M.; Begley, T.H.; Krynitsky, A.J.; Rader, J.I. Determination of multiple mycotoxins in dietary supplements containing green coffee bean extracts using ultrahigh-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS). J. Agric. Food Chem. 2013, 61, 4822–4830. [Google Scholar] [CrossRef]
- Napolitano, A.; Fogliano, V.; Tafuri, A.; Ritieni, A. Natural occurrence of ochratoxin A and antioxidant activities of green and roasted coffees and corresponding byproducts. J. Agric. Food Chem. 2007, 55, 10499–10504. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-DeHond, A.; Haza, A.I.; Ávalos, A.; del Castillo, M.D.; Morales, P. Validation of coffee silverskin extract as a food ingredient by the analysis of cytotoxicity and genotoxicity. Food Res. Int. 2017, 100, 791–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toschi, T.G.; Cardenia, V.; Bonaga, G.; Mandrioli, M.; Rodriguez-Estrada, M.T. Coffee silverskin: Characterization, possible uses, and safety aspects. J. Agric. Food Chem. 2014, 62, 10836–10844. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Gaspar, C.; Palmeira-de-Oliveira, A.; Sarmento, B.; Helena Amaral, M.; P P Oliveira, M.B. Application of coffee silverskin in cosmetic formulations: Physical/antioxidant stability studies and cytotoxicity effects. Drug Dev. Ind. Pharm. 2016, 42, 99–106. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Rios, M.B.; Herrera, T.; Rodriguez-Bertos, A.; Nuñez, F.; San Andres, M.I.; Sanchez-Fortun, S.; Del Castillo, M.D. Coffee silverskin extract: Nutritional value, safety and effect on key biological functions. Nutrients 2019, 11, 2693. [Google Scholar] [CrossRef] [Green Version]
- Mussatto, S.I.; Carneiro, L.M.; Silva, J.P.A.; Roberto, I.C.; Teixeira, J.A. A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohydr. Polym. 2011, 83, 368–374. [Google Scholar] [CrossRef] [Green Version]
- Del Castillo, M.D.; Fernandez-Gomez, B.; Martinez-Saez, N.; Iriondo-DeHond, A.; Mesa, M.D. Coffee By-products (chapter 12). In Coffee: Production, Quality and Chemistry; Farah, A., Ed.; Royal Society of Chemistry: London, UK, 2019; pp. 309–334. [Google Scholar]
- Janissen, B.; Huynh, T. Chemical composition and value-adding applications of coffee industry by-products: A. review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Cornejo, F.S.; Fernandez-Gomez, B.; Vera, G.; Guisantes-Batan, E.; Alonso, S.G.; Andres, M.I.S.; Sanchez-Fortun, S.; Lopez-Gomez, L.; Uranga, J.A.; et al. Bioaccessibility, metabolism, and excretion of lipids composing spent coffee grounds. Nutrients 2019, 11, 1411. [Google Scholar] [CrossRef] [Green Version]
- Ramalakshmi, K.; Rao, L.J.M.; Takano-Ishikawa, Y.; Goto, M. Bioactivities of low-grade green coffee and spent coffee in different in vitro model systems. Food Chem. 2009, 115, 79–85. [Google Scholar] [CrossRef]
- Andrade, K.S.; Gonçalvez, R.T.; Maraschin, M.; Ribeiro-do-Valle, R.M.; Martínez, J.; Ferreira, S.R.S. Supercritical fluid extraction from spent coffee grounds and coffee husks: Antioxidant activity and effect of operational variables on extract composition. Talanta 2012, 88, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W.; Schwarz, S.; Teipel, J.; Hegmanns, M.; Kuballa, T.; Walch, S.G.; Breitling-Utzmann, C.M. Potential antagonistic effects of acrylamide mitigation during coffee roasting on furfuryl alcohol, furan and 5-hydroxymethylfurfural. Toxics 2018, 7, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickard, S.; Wilms, H.; Richling, E. Alkylpyrazine contents of coffee beverages using stable isotope dilution gas chromatography–mass spectrometry. LWT Food Sci. Technol. 2014, 58, 188–193. [Google Scholar] [CrossRef]
- Kremer, J.I.; Pickard, S.; Stadlmair, L.F.; Glaß-Theis, A.; Buckel, L.; Bakuradze, T.; Eisenbrand, G.; Richling, E. Alkylpyrazines from coffee are extensively metabolized to pyrazine carboxylic acids in the human body. Mol. Nut. Food Res. 2019, e1801341. [Google Scholar] [CrossRef] [Green Version]
- Shortt, J. A Hand-Book to Coffee Planting in Southern India; Pharoah and Co.: Chingleput, India, 1864. [Google Scholar]
- Elliot, R.H. The Experiences of a Planter in the Jungles of Mysore; Chapman and Hall: London, UK, 1871. [Google Scholar]
- McCabe Baghdiantz, I. Orientalism in early modern France. In Eurasian Trade, Exoticism and the Ancien Régime; Berg: Oxford, UK; New York, NY, USA, 2008. [Google Scholar]
- Neumann, C. Lectiones Publicae von vier Subiectis Diaeteticis: Vom Thée, Caffée, Bier, und Wein; Palala Press: Berlin, Germany, 1735. [Google Scholar]
- Naturforschende Gesellschaft in Danzig. Versuche und Abhandlungen der Naturforschende Gesellschaft in Danzig, Teil 3; Schreiberische Buchdruckerei: Danzig, Poland, 1756. [Google Scholar]
- European Union. Human Consumption to a Significant Degree; Information and Guidance Document; European Union: Brussels, Belgium, 2012; Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/novel-food_guidance_human-consumption_en.pdf (accessed on 9 April 2020).
- AM Breweries IVS. Herbal Infusion Made from Coffee Leaves. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/novel-food_sum_ongoing-not_2018-0740.pdf (accessed on 16 March 2020).
- Panama Varietals GmbH. Coffee Husk (Cascara)—The Dried Husk of the Coffee Fruit or Coffee Cherry. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/novel-food_sum_ongoing-app_2018-0192.pdf (accessed on 20 March 2020).
- European Union. Summary of Applications and Notifications. Available online: https://ec.europa.eu/food/safety/novel_food/authorisations/summary-applications-and-notifications_en (accessed on 9 April 2020).
- European Union. Commission implementing regulation (EU) 2018/456 of 19 March 2018 on the procedural steps of the consultation process for determination of novel food status in accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. Off. J. Eur. Union 2018, L77, 6–13. [Google Scholar]



| Coffee By-Product | Possible Use as Food | |
|---|---|---|
| Flowers |  | Beverages (tea): “coffee blossom tea” [16] |
| Leaves |  | Beverages (tea): “coffee leaf tea” [17,18,19,20,21] |
| Coffee pulp |  | Jam, juice, concentrate, jelly [22]; Coffee pulp flour for breads, cookies, muffins, squares, brownies, pastas, sauces and beverages [23]; spirits/ethanol [24] |
| Husks, cascara, dried coffee cherries |  | Beverages (tea) [25,26,27]; spirits [28,29]; qishr (mixture with spices) [30,31]; dietary fiber source [11,32]; extraction of caffeine [24] |
| Green unroasted beans |  | Dietary supplement [33,34,35]; beverages (tea): “white coffee” [36,37] |
| Silver skin |  | Dietary fiber source [38,39,40,41,42,43]; bakery products (breads, biscuits) [44,45,46,47]; beverages (tea) [48,49]; smoke flavor additive |
| Spent coffee grounds |  | Historical: adulteration of coffee; spirits [50]; bakery products [51,52,53]; dietary fiber source [54,55] |
| Defatted Coffea arabica seed powder (coffee flour) for savory and sweet recipes, in bakery, confectionary, snacks, ready-to-eat products [56] | ||
| Parchment |  | Food preservative, antioxidant [24,57] |
| Coffee By-Product | Novel Food Status a |
|---|---|
| Flowers | Novel. Probably not a traditional food from third country. Needs approval procedure. |
| Leaves | Novel, notification for infusion from coffee leaves as traditional food from third country (Ethiopia) submitted by AM Breweries IVS, Amager, Denmark [146]. |
| Coffee pulp | Novel, currently not approved. No application pending. Needs approval procedure. |
| Husks, cascara, dried coffee cherries | Novel, application submitted by Panama Varietals GmbH, Marchtrenk, Austria [147]. |
| Green unroasted beans | Not novel [10]. The classification also applies to the non-selective water extraction made of them. Selective extracts could be novel. |
| Silver skin | Unclear. Consultation procedure suggested. |
| Used coffee grounds | Novel, currently not approved. No application pending. Needs approval procedure. |
| Novel, application submitted for a certain “coffee flour” by Kaffee Bueno ApS, Copenhagen, Denmark [56]. | |
| Stems, twigs, wood | Non-food material, contamination up to certain levels typically tolerated in the trade of green coffee. |
| Parchment | Novel, currently not approved. No application pending. Needs approval procedure. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klingel, T.; Kremer, J.I.; Gottstein, V.; Rajcic de Rezende, T.; Schwarz, S.; Lachenmeier, D.W. A Review of Coffee By-Products Including Leaf, Flower, Cherry, Husk, Silver Skin, and Spent Grounds as Novel Foods within the European Union. Foods 2020, 9, 665. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9050665
Klingel T, Kremer JI, Gottstein V, Rajcic de Rezende T, Schwarz S, Lachenmeier DW. A Review of Coffee By-Products Including Leaf, Flower, Cherry, Husk, Silver Skin, and Spent Grounds as Novel Foods within the European Union. Foods. 2020; 9(5):665. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9050665
Chicago/Turabian StyleKlingel, Tizian, Jonathan I. Kremer, Vera Gottstein, Tabata Rajcic de Rezende, Steffen Schwarz, and Dirk W. Lachenmeier. 2020. "A Review of Coffee By-Products Including Leaf, Flower, Cherry, Husk, Silver Skin, and Spent Grounds as Novel Foods within the European Union" Foods 9, no. 5: 665. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9050665