Can Manipulation of Durum Wheat Amylose Content Reduce the Glycaemic Index of Spaghetti?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Sample Preparation and Analytical Methods
2.3. Pasta Preparation and Evaluation
2.4. In Vitro Starch Digestion of Pasta
2.5. In Vivo Glycaemic Index (GI) Testing
2.6. Statistical Methods
3. Results and Discussion
3.1. Impact of Elevated Amylose on Pasta Technological Properties
3.2. Impact of Elevated Amylose on Pasta In Vitro Starch Digestion
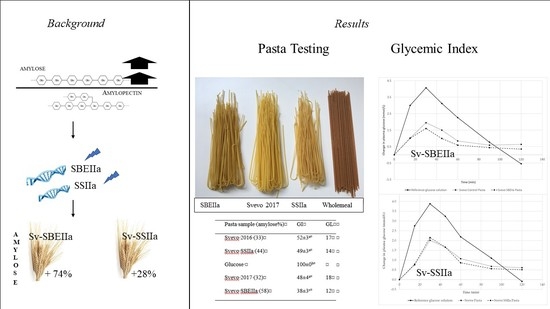
3.3. Impact of Elevated Amylose on Pasta In Vivo Starch Digestion and Glycaemic Index
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prückler, M.; Siebenhandl-Ehn, S.; Apprich, S.; Höltinger, S.; Haas, C.; Schmid, E.; Kneifel, W. Wheat bran-based biorefinery 1: Composition of wheat bran and strategies of functionalization. LWT 2014, 56, 211–221. [Google Scholar] [CrossRef]
- Björck, I.; Östman, E.; Kristensen, M.; Anson, N.M.; Price, R.K.; Haenen, G.R.; Havenaar, R.; Knudsen, K.E.B.; Frid, A.; Mykkänenh, H.; et al. Cereal grains for nutrition and health benefits: Overview of results from in vitro, animal and human studies in the HEALTHGRAIN project. Trends Food Sci. Tech. 2012, 25, 87–100. [Google Scholar] [CrossRef]
- Zong, G.; Gao, A.; Hu, F.B.; Sun, Q. Whole grain intake and mortality from all causes, cardiovascular disease, and cancer a meta-analysis of prospective cohort studies. Circulation 2016, 133, 2370–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.M. CODEX-aligned dietary fiber definitions help to bridge the ‘fiber gap’. Nut. J. 2014, 13, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grigor, J.M.; Brennan, C.S.; Hutchings, S.C.; Rowlands, D.S. The sensory acceptance of fibre-enriched cereal foods: A meta-analysis. Int. J. Food Sci. Technol. 2016, 51, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Mercier, S.; Moresoli, C.; Mondor, M.; Villeneuve, S.; Marcos, B. A meta-analysis of enriched pasta: What are the effects of enrichment and process specifications on the quality attributes of pasta? Compr. Rev. Food Sci. Food Saf. 2016, 15, 685–704. [Google Scholar] [CrossRef] [Green Version]
- Bird, A.R.; Regina, A. High amylose wheat: A platform for delivering human health benefits. J. Cereal Sci. 2018, 82, 99–105. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant starch—A review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef]
- Aravind, N.; Sissons, M.; Fellows, C.M.; Blazek, J.; Gilbert, E.P. Optimisation of resistant starch II and III levels in durum wheat pasta to reduce in vitro digestibility while maintaining processing and sensory characteristics. Food Chem. 2013, 136, 1100–1109. [Google Scholar] [CrossRef]
- Bustos, M.C.; Perez, G.T.; Leon, A.E. Sensory and nutritional attributes of fibre-enriched pasta. LWT 2011, 44, 1429–1434. [Google Scholar] [CrossRef]
- Gelencsér, T.; Gál, V.; Hódsagi, M.; Salgó, A. Evaluation of quality and digestibility characteristics of resistant starch-enriched pasta. Food Bioprocess Tec. 2008, 1, 171–179. [Google Scholar] [CrossRef]
- Sozer, N.; Dalgic, A.C.; Kaya, A. Thermal, textural and cooking properties of spaghetti enriched with resistant starch. J. Food Eng. 2007, 81, 476–484. [Google Scholar] [CrossRef]
- Berry, C.S. Resistant starch. Formation and measurement of starch that survives exhaustive digestion with amylolytic enzymes during the determination of dietary fiber. J. Cereal Sci. 1986, 4, 301–314. [Google Scholar] [CrossRef]
- Li, H.; Gidley, M.J.; Dhital, S. High-amylose starches to bridge the “fiber gap”: Development, structure, and nutritional functionality. Compr. Rev. Food Sci. Food Saf. 2019, 18, 362–379. [Google Scholar] [CrossRef] [Green Version]
- Regina, A.; Bird, A.; Topping, D.; Bowden, S.; Freeman, J.; Barsby, T.; Kosar-Hashemi, B.; Li, Z.; Rahman, S.; Morell, M. High-amylose wheat generated by RNA interference improves indices of large-bowel health in rats. Proc. Natl. Acad. Sci. USA 2006, 103, 3546–3551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slade, A.J.; Fuerstenberg, S.I.; Loeffler, D.; Steine, M.N.; Facciotti, D. A reverse genetic, nontransgenic approach to wheat crop improvement by TILLING. Nat. Biotechnol. 2005, 23, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Slade, A.J.; McGuire, C.; Loeffler, D.; Mullenberg, J.; Skinner, W.; Fazio, G.; Knauf, V.C. Development of high amylose wheat through TILLING. BMC Plant Biol. 2012, 12, 69. [Google Scholar] [CrossRef] [Green Version]
- Sestili, F.; Janni, M.; Doherty, A.; Botticella, E.; D’Ovidio, R.; Masci, S.; Jones, H.D.; Lafiandra, D. Increasing the amylose content of durum wheat through silencing of the SBEIIa genes. BMC Plant Biol. 2010, 10, 144. [Google Scholar] [CrossRef] [Green Version]
- Sestili, F.; Palombieri, S.; Botticella, E.; Mantovani, P.; Bovina, R.; Lafiandra, D. TILLING mutants of durum wheat result in a high amylose phenotype and provide information on alternative splicing mechanisms. Plant Sci. 2015, 233, 127–133. [Google Scholar] [CrossRef]
- Botticella, E.; Sestili, F.; Sparla, F.; Moscatello, S.; Marri, L.; Cuesta-Seijo, J.A.; Falini, G.; Battistelli, A.; Trost, P.; Lafiandra, D. Combining mutations at genes encoding key enzymes involved in starch synthesis affects the amylose content, carbohydrate allocation and hardness in the wheat grain. Plant Biotechnol. J. 2018, 16, 1723–1734. [Google Scholar] [CrossRef] [Green Version]
- Hazard, B.; Zhang, X.; Colasuonno, P.; Uauy, C.; Beckles, D.M.; Dubcovsky, J. Induced mutations in the starch branching enzyme II (SBEII) genes increase amylose and resistant starch content in durum wheat. Crop Sci. 2012, 52, 1754–1766. [Google Scholar] [PubMed]
- Botticella, E.; Sestili, F.; Ferrazzano, G.; Mantovani, P.; Cammerata, A.; D’Egidio, M.G.; Lafiandra, D. The impact of the SSIIa null mutations on grain traits and composition in durum wheat. Breed. Sci. 2016, 66, 572–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogg, A.C.; Gause, K.; Hofer, P.; Martin, J.M.; Graybosch, R.A.; Hansen, L.E.; Giroux, M.J. Creation of a high-amylose durum wheat through mutagenesis of starch synthase II (SSIIa). J. Cereal Sci. 2013, 57, 377–383. [Google Scholar] [CrossRef] [Green Version]
- Hogg, A.C.; Martin, J.M.; Manthey, F.A.; Giroux, M.J. Nutritional and quality traits of pasta made from SSIIa null high-amylose durum wheat. Cereal Chem. 2015, 92, 395–400. [Google Scholar] [CrossRef]
- AACC International. Approved Methods of Analysis, 11th ed.; Method 26-41.01., 44-15A., [25]54-70.01., 66-51.01; AACC International: St. Paul, MN, USA, 2010. [Google Scholar]
- Horneck, D.A.; Miller, R.O. Determination of total nitrogen in plant tissue. Handb. Ref. Methods Plant Anal. 1998, 2, 75–83. [Google Scholar]
- Sharma, R.; Sissons, M.J.; Rathjen, A.J.; Jenner, C.F. The null-4A allele at the waxy locus in durum wheat affects pasta cooking quality. J. Cereal Sci. 2002, 35, 287–297. [Google Scholar] [CrossRef]
- Li, H.; Dhital, S.; Slade, A.J.; Yu, W.; Gilbert, R.G.; Gidley, M.J. Altering starch branching enzymes in wheat generates high-amylose starch with novel molecular structure and functional properties. Food Hydrocoll. 2019, 92, 51–59. [Google Scholar] [CrossRef]
- Sissons, M.; Ovenden, B.; Adorada, D.; Milgate, A. Durum wheat quality in high input irrigation systems in south eastern Australia. Crop Pasture Sci. 2014, 65, 411–422. [Google Scholar] [CrossRef]
- Morita, N.; Maeda, T.; Miyazaki, M.; Yamammori, M.; Miura, H.; Phtsuka, I. Dough and baking properties of high amylose and waxy wheat flours. Cereal Chem. 2002, 79, 491–495. [Google Scholar] [CrossRef]
- Sissons, M.J.; Aravind, N.; Fellows, C.M. Quality of fibre-enriched spaghetti containing microbial transglutaminase. Cereal Chem. 2010, 87, 57–64. [Google Scholar] [CrossRef]
- Zou, W.; Sissons, M.; Gidley, M.J.; Gilbert, R.G.; Warren, F.J. Combined techniques for characterising pasta structure reveals how the gluten network slows enzymic digestion rate. Food Chem. 2015, 188, 559–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vansteelandt, J.; Delcour, J.A. Characterisation of starch from durum wheat (Triticum durum). Starch-Stärke 1999, 51, 73–80. [Google Scholar] [CrossRef]
- Hogg, A.C.; Martin, J.M.; Giroux, M.J. Novel ssIIa alleles produce specific seed amylose levels in hexaploid wheat. Cereal Chem. 2017, 94, 1008–1015. [Google Scholar] [CrossRef]
- Rakszegi, M.; Kisgyörgy, B.N.; Kiss, T.; Sestili, F.; Láng, L.; Lafiandra, D.; Bedő, Z. Development and characterization of high-amylose wheat lines. Starch-Stärke 2015, 67, 247–254. [Google Scholar] [CrossRef]
- Konik-Rose, C.; Thistleton, J.; Chanvrier, H.; Tan, I.; Halley, P.; Gidley, M.; Kosar-Hashemi, B.; Wang, H.; Larroque, O.; Ikea, J.; et al. Effects of starch synthase IIa gene dosage on grain, protein and starch in endosperm of wheat. Theor. Appl. Genet. 2007, 115, 1053–1062. [Google Scholar] [CrossRef]
- Soh, H.N.; Sissons, M.J.; Turner, M.A. Effect of starch granule size distribution and elevated amylose content on durum dough rheology and spaghetti cooking quality. Cereal Chem. 2006, 83, 513–519. [Google Scholar] [CrossRef]
- Tester, R.F.; Morrison, W.R. Swelling and gelatinization of cereal starches. I. Effects of amylopectin, amylose, and lipids. Cereal Chem. 1990, 67, 551–557. [Google Scholar]
- Tomoko, S.; Matsuki, J. Effect of wheat starch structure on swelling power. Cereal Chem. 1998, 75, 525–529. [Google Scholar]
- Dick, J.W.; Youngs, V.L. Evaluation of durum wheat, semolina, and pasta in the United States. In Durum Wheat: Chemistry and Technology; Fabriani, G., Lintas, C., Eds.; American Association of Cereal Chemists: St. Paul, MN, USA, 1988; pp. 237–248. [Google Scholar]
- Clarke, F.R.; Clarke, J.M.; McCaig, T.N.; Know, R.E.; DePauw, R.M. Inheritance of yellow pigment concentration in seven durum wheat crosses. Can. J. Plant Sci. 2006, 86, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Kovacs, M.I.P.; Fowler, D.B.; Holley, R. Effects of protein content and composition on white noodle making quality: Color. Cereal Chem. 2004, 81, 777–784. [Google Scholar] [CrossRef]
- Hazard, B.; Zhang, X.; Naemeh, R.; Hamilton, M.K.; Rust, B.; Raybould, H.E.; Newman, J.W.; Martin, R.; Dubcovsky, J. Mutations in durum wheat SBEII genes conferring increased amylose and resistant starch affect grain yield components, semolina and pasta quality and fermentation responses in rats. Crop Sci. 2015, 55, 2813–2825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sozer, N.; Kaya, A. The effect of cooking water composition on textural and cooking properties of spaghetti. Int. J. Food Prop. 2008, 11, 351–362. [Google Scholar] [CrossRef]
- Edwards, N.M.; Izydorczyk, M.S.; Dexter, J.E.; Biliaderis, C.G. Cooked pasta texture: Comparison of dynamic viscoelastic properties to instrumental assessment of firmness. Cereal Chem. 1993, 70, 122–126. [Google Scholar]
- Sissons, M.J.; Egan, N.E.; Gianibelli, M.C. New insights into the role of gluten on durum pasta quality using reconstitution method. Cereal Chem. 2005, 82, 601–608. [Google Scholar] [CrossRef]
- Martin, J.M.; Hogg, A.C.; Hofer, P.; Manthey, F.A.; Giroux, M.J. Impacts of SSIIa-A null allele on durum wheat noodle quality. Cereal Chem. 2014, 91, 176–182. [Google Scholar] [CrossRef]
- Martin, J.M.; Talbert, L.E.; Habernicht, D.K.; Lanning, S.P.; Sherman, J.D.; Carlson, G.; Giroux, M.J. Reduced amylose effects on bread and white salted noodle quality. Cereal Chem. 2004, 81, 188–193. [Google Scholar] [CrossRef]
- Gianibelli, M.C.; Sissons, M.J.; Batey, I.L. Effect of different waxy starches on pasta cooking quality of durum wheat. Cereal Chem. 2005, 82, 321–327. [Google Scholar] [CrossRef]
- Haralampu, S.G. Resistant starch—A review of the physical properties and biological impact of RS3. Carbohydr. Polym. 2000, 41, 285–292. [Google Scholar] [CrossRef]
- Newberry, M.; Berbezy, P.; Belobrajdic, D.; Chapron, S.; Tabouillot, P.; Regina, A.; Bird, A. High-amylose wheat foods: A new opportunity to meet dietary fiber targets for health. Cereal Foods World 2018, 63, 188–193. [Google Scholar]
- Evans, A.; Thompson, D.B. Resistance to α-amylase digestion in four native high-amylose maize starches. Cereal Chem. 2004, 81, 31–37. [Google Scholar] [CrossRef]
- Tester, R.F.; Qi, X.; Karkalas, J. Hydrolysis of native starches with amylases. Anim. Feed Sci. Tech. 2006, 130, 39–54. [Google Scholar] [CrossRef]
- Fardet, A.; Hoebler, C.; Baldwin, P.M.; Bouchet, B.; Gallant, D.J.; Barry, J.L. Involvement of the protein network in the in vitro degradation of starch from spaghetti and lasagne: A microscopic and enzymic study. J. Cereal Sci. 1998, 27, 133–145. [Google Scholar] [CrossRef]
- Hoebler, C.; Karinthi, A.; Chiron, H.; Champ, M.; Barry, J.L. Bioavailability of starch in bread rich in amylose: Metabolic responses in healthy subjects and starch structure. Eur. J. Clin. Nutr. 1999, 53, 360–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrado, M.; Cherta-Murillo, A.; Chambers, E.S.; Wood, A.J.; Plummer, A.; Lovegrove, A.; Edwards, C.H.; Frost, G.S.; Hazard, B.A. Effect of semolina pudding prepared from starch branching enzyme IIa and b mutant wheat on glycaemic response in vitro and in vivo: A randomised controlled pilot study. Food Funct. 2020, 11, 617–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mir, J.A.; Srikaeo, K.; García, J. Effects of amylose and resistant starch on starch digestibility of rice flours and starches. Int. Food Res. J. 2013, 20, 1329–1335. [Google Scholar]
- Svihus, B.; Uhlen, A.K.; Harstad, O.M. Effect of starch granule structure, associated components and processing on nutritive value of cereal starch: A review. Anim. Feed Sci. Tech. 2005, 122, 303–320. [Google Scholar] [CrossRef]
- Joint FAO/WHO Report. Carbohydrates in Human Nutrition; FAO Food and Nutrition, 1998; p. 66. Available online: https://www.who.int/nutrition/publications/nutrientrequirements/9251041148/en/ (accessed on 27 May 2020).
- Vetrani, C.; Sestili, F.; Vitale, M.; Botticella, E.; Giacco, R.; Griffo, E.; Costabile, G.; Cipriano, P.; Tura, A.; Pacini, G.; et al. Metabolic response to amylose-rich wheat-based rusks in overweight individuals. Eur. J. Clin. Nutr. 2018, 72, 904–912. [Google Scholar] [CrossRef]
- Belobrajdic, D.P.; Regina, A.; Klingner, B.; Zajac, I.; Chapron, S.; Berbezy, P.; Bird, A.R. High-amylose wheat lowers the postprandial glycemic response to bread in healthy adults: A randomized controlled crossover trial. J. Nutr. 2019, 149, 1335–1345. [Google Scholar] [CrossRef]
- Edwards, C.H.; Cochetel, N.; Setterfield, L.; Perez-Moral, N.; Warren, F.J. A single-enzyme system for starch digestibility screening and its relevance to understanding and predicting the glycaemic index of food products. Food Funct. 2019, 10, 4751–4760. [Google Scholar] [CrossRef] [Green Version]
- Chiavaroli, L.; Kendall, C.W.C.; Braunstein, C.R.; Blanco Mejia, S.; Leiter, L.A.; Jenkins, D.J.A.; Sievenpiper, J.L. Effect of pasta in the context of low-glycaemic index dietary patterns on body weight and markers of adiposity: A systematic review and meta-analysis of randomised controlled trials in adults. BMJ Open 2018, 8, e019438. [Google Scholar] [CrossRef] [Green Version]




| Sample | SP | Amylose (%) | Protein (11%mb) a | Ash (14%mb) | FWA (14%mb) | TDF (%) | TIF (%) | TSF (%) | Granularity (g/100 g) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 500 µm | 425 µm | 315 µm | 250 µm | 180 µm | <180 µm | |||||||||
| Svevo2016 | 8.93 ± 0.61 a | 34.0 ± 1. 7 a | 12.9 | 0.70 ± 0.049 | 59.4 ± 0.14 a | nd | nd | nd | 0.07 | 4.1 | 33.8 | 27.5 | 18.6 | 15.3 |
| Svevo SSIIa | 6.62 ± 0.69 b | 43.5 ± 1.5 b | 14.2 | 0.82 ± 0.014 | 74.3 ± 0.28 b | nd | nd | nd | 0.01 | 2.0 | 35.3 | 39.1 | 17.8 | 5.4 |
| Svevo2017 | 10.56 ± 0.59 a | 33.3 ± 1.3 c | 12.8 | 0.53 ± 0.012 | 57.0 ± 0.14 c | 2.5 a | 1.4 a | 1.1 a | 0.01 | 0.06 | 43.2 | 36.1 | 17.4 | 2.7 |
| Svevo SBEIIa | 5.14 ± 0.45 b | 57.8 ± 1.5 d | 15.4 | 0.81 ± 0.042 | 77.2 ± 0.21 d | 5.7 b | 3.8 b | 1.9 b | 0.02 | 0.02 | 44.3 | 34.1 | 17.9 | 3.3 |
| Sample | Field Season | Dry Pasta | ||||
|---|---|---|---|---|---|---|
| DP-L * | DP-a * | DP-b * | RS% (dm) | TS% (dm) | ||
| Svevo | 2016 | 70.12 ± 0.30 a | 0.29 ± 0.14 a | 44.59 ± 0.62 a | 0.73 ± 0.01 a | 73.4 ± 0.06 a |
| Svevo SSIIa | 2016 | 67.01 ± 0.68 b | 2.13 ± 0.66 b | 38.31 ± 1.05 b | 2.06 ± 0.01 b | 67.3 ± 1.42 b |
| Svevo | 2017 | 71.57 ± 0.17 c | −1.95 ± 0.05 c | 49.06 ± 0.45 c | 0.21 ± 0.02 c | 75.6 ± 0.78 a |
| Svevo SBEIIa | 2017 | 64.49 ± 0.92 d | 1.53 ± 0.27 d,b | 39.99 ± 1.36 d | 7.36 ± 0.10 d | 66.4 ± 0.50 b |
| Commercial wholemeal | 44.66 | 15.25 | 18.66 | nd | nd | |
| Pasta | Field Season | FCT (s) | Firmness | Overcook Tolerance | Stickiness | Cooking Loss (%) | Water Absorption | |||
|---|---|---|---|---|---|---|---|---|---|---|
| PH (g) | Area (g/s) | PH/P | PH (g) | Area (g/s) | ||||||
| Svevo | 2016 | 661 ± 50 | 1334 ± 65 a | 615 ± 22 a | 103 ± 5.1 a | 52 a | 17.0 ± 1.8 a,b | 9.3 ± 0.68 a | 4.6 ± 0.11 a | 146 ± 2.96 a |
| Svevo SSIIa | 2016 | 578 ± 20 | 1139 ± 82 b | 566 ± 51 b | 80 ± 5.8 b | 52 a | 14.7 ± 1.5 a | 5.4 ± 0.83 b | 6.9 ± 0.01 b | 123 ± 0.01 b |
| Svevo | 2017 | 761 ± 19 | 1261 ± 37 c | 539 ± 16 c | 99 ± 2.9 c | 54 b | 18.7 ± 1.5 b | 6.8 ± 2.2 c,b | 4.8 ± 0.30 a | 158 ± 0.38 c |
| Svevo SBEIIa | 2017 | 678 ± 40 | 1124 ± 82 b | 544 ± 51 c | 73 ± 5.3 d | 49 c | 15.9 ± 1.3 a | 5.7 ± 1.0 c,b | 6.6 ± 0.14 b | 120 ± 1.53 b |
| Tonset (°C) | Tpeak (°C) | Tend (°C) | Enthalpy (J/kg) | |
|---|---|---|---|---|
| Svevo 2017 | 52.76 ± 0.46 b,c | 59.51 ± 0.47 a,b | 66.78 ± 0.05 a | 11.71 ± 0.38 b,c |
| Svevo SBEIIa | 52.27 ± 0.19 b | 65.49 ± 0.23 d | 88.83 ± 0.23 d | 7.92 ± 1.40 a,b |
| Svevo 2016 | 53.66 ± 0.56 c | 59.91 ± 0.19 b,c | 67.81 ± 0.53 a,b | 10.91 ± 1.42 a,b,c |
| Svevo SSIIa | 47.04 ± 0.08 a | 60.93 ± 0.40 c | 69.57 ± 0.18 c | 7.23 ± 1.50 a |
| Pasta | Total Area under Digestion Curve | Normalised Area | C∞ % I | k1 | C∞ % II | k2 |
|---|---|---|---|---|---|---|
| Svevo 2016 | 19,851 a | 1.00 | 38.9 | 0.02756 | 83.2 | 0.00654 |
| Svevo SSIIa | 18,652 b | 0.94 | 35.4 | 0.02554 | 81.0 | 0.00574 |
| Svevo 2017 | 20,572 a | 1.04 (1.00) | 37.2 | 0.02670 | 87.1 | 0.00615 |
| Svevo SBEIIa | 12231 c | 0.62 (0.59) | 24.3 | 0.03470 | 45.2 | 0.00831 |
| Test Food | Available Carbohydrates for 100 Grams (g) | Portion Size (g) | Available Carbohydrates in Test Portion (g) |
|---|---|---|---|
| Glucose (ref.) | 97.30 | 51.4 g glucose 250 mL water | 50.0 |
| Svevo 2016 | 64.19 | 77.9 g dry pasta | 50.0 |
| Svevo SSIIa | 57.35 | 87.2 g dry pasta | 50.0 |
| Svevo 2017 | 74.05 | 67.5 g dry pasta | 50.0 |
| Svevo SBEIIa | 65.27 | 76.6 g dry pasta | 50.0 |
| Pasta Sample (Amylose%) | GI | GL |
|---|---|---|
| Svevo 2016 (33) | 52 ± 3 a | 17 |
| Svevo SSIIa (44) | 49 ± 3 a | 14 |
| Glucose | 100 ± 0 b | |
| Svevo 2017 (32) | 48 ± 4 a | 18 |
| Svevo SBEIIa (58) | 38 ± 3 c | 12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sissons, M.; Sestili, F.; Botticella, E.; Masci, S.; Lafiandra, D. Can Manipulation of Durum Wheat Amylose Content Reduce the Glycaemic Index of Spaghetti? Foods 2020, 9, 693. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9060693
Sissons M, Sestili F, Botticella E, Masci S, Lafiandra D. Can Manipulation of Durum Wheat Amylose Content Reduce the Glycaemic Index of Spaghetti? Foods. 2020; 9(6):693. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9060693
Chicago/Turabian StyleSissons, Mike, Francesco Sestili, Ermelinda Botticella, Stefania Masci, and Domenico Lafiandra. 2020. "Can Manipulation of Durum Wheat Amylose Content Reduce the Glycaemic Index of Spaghetti?" Foods 9, no. 6: 693. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9060693