Characterization and Antimicrobial Activity of Biodegradable Active Packaging Enriched with Clove and Thyme Essential Oil for Food Packaging Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
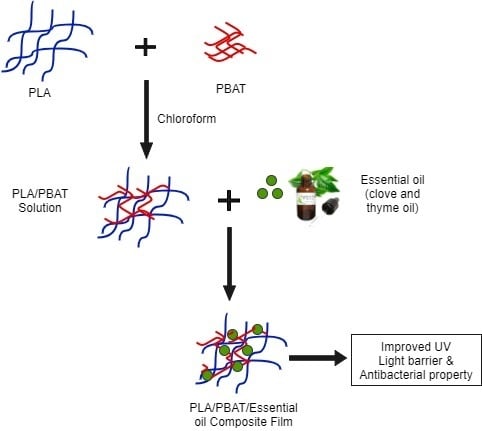
2.2. Preparation of Composite Films
2.3. Characterisation of Films
2.3.1. Surface Colour and Optical Properties
2.3.2. Thickness and Tensile Properties
2.3.3. Surface Hydrophobicity
2.3.4. Antibacterial Activity
2.3.5. Biofilm Inhibition
2.4. Statistical Analysis
3. Results and Discussion
3.1. Surface Colour
3.2. Optical Properties
3.3. FTIR Analysis
3.4. Thickness of the Films
3.5. Tensile Properties of the Films
3.6. Water Contact Angle (WCA)
3.7. Antibacterial Activity
3.8. Biofilm Inhibition
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schaefer, D.; Cheung, W.M. Smart packaging: Opportunities and challenges. Procedia CIRP 2018, 72, 1022–1027. [Google Scholar] [CrossRef]
- Weng, Y.X.; Jin, Y.J.; Meng, Q.Y.; Wang, L.; Zhang, M.; Wang, Y.Z. Biodegradation behavior of poly (butylene adipate-co-terephthalate) (PBAT), poly (lactic acid) (PLA), and their blend under soil conditions. Polym. Test. 2013, 32, 918–926. [Google Scholar] [CrossRef]
- Brockgreitens, J.; Abbas, A. Responsive food packaging: Recent progress and technological prospects. Compr. Rev. Food Sci. 2016, 15, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Azadbakht, E.; Maghsoudlou, Y.; Khomiri, M.; Kashiri, M. Development and structural characterization of chitosan films containing Eucalyptus globulus essential oil: Potential as an antimicrobial carrier for packaging of sliced sausage. Food Packag. Shelf Life 2018, 17, 65–72. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; de Melo, N.R.; Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Vinceković, M.; Viskić, M.; Jurić, S.; Giacometti, J.; Kovačević, D.B.; Putnik, P.; Jambrak, A.R. Innovative technologies for encapsulation of Mediterranean plants extracts. Trends Food Sci. Technol. 2017, 69, 1–12. [Google Scholar] [CrossRef]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Kovačević, D.B.; Pateiro, M.; Lorenzo, J.M. Active packaging films with natural antioxidants to be used in meat industry: A review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Y.; Cui, H. Electrospun thyme essential oil/gelatin nanofibers for active packaging against Campylobacter jejuni in chicken. LWT 2018, 97, 711–718. [Google Scholar] [CrossRef]
- Perdones, Á.; Escriche, I.; Chiralt, A.; Vargas, M. Effect of chitosan–lemon essential oil coatings on volatile profile of strawberries during storage. Food Chem. 2016, 197, 979–986. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Stoilova, I.; Stoyanova, A.; Krastanov, A.; Schmidt, E. Chemical composition and antioxidant properties of clove leaf essential oil. J. Agric. Food Chem 2006, 54, 6303–6307. [Google Scholar] [CrossRef]
- Pinto, E.; Vale-Silva, L.; Cavaleiro, C.; Salgueiro, L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2009, 58, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Srivastava, S.K.; Syamsundar, K.V. Bud and leaf essential oil composition of Syzygium aromaticum from India and Madagascar. Flav. Frag. J. 2005, 20, 51–53. [Google Scholar] [CrossRef]
- Razafimamonjison, G.; Jahiel, M.; Duclos, T.; Ramanoelina, P.; Fawbush, F.; Danthu, P. Bud, leaf and stem essential oil composition of Syzygium aromaticum from Madagascar, Indonesia and Zanzibar. Int. J. Basic Appl. Sci. 2014, 3, 224. [Google Scholar]
- Nzeako, B.; Al-Kharousi, Z.S.; Al-Mahrooqui, Z. Antimicrobial activities of clove and thyme extracts. Sultan Qaboos Univ. Med. J. 2006, 6, 33. [Google Scholar]
- Porte, A.; Godoy, R.L. Chemical composition of Thymus vulgaris L. (Thyme) essential oil from the Rio de Janeiro state, Brazil. J. Serbian Chem. Soc. 2008, 73, 307–310. [Google Scholar] [CrossRef]
- Gedikoğlu, A.; Sökmen, M.; Çivit, A. Evaluation of Thymus vulgaris and Thymbra spicata essential oils and plant extracts for chemical composition, antioxidant, and antimicrobial properties. Food Sci. Nutr. 2019, 7, 1704–1714. [Google Scholar] [CrossRef] [Green Version]
- Imelouane, B.; Amhamdi, H.; Wathelet, J.P.; Ankit, M.; Khedid, K.; El Bachiri, A. Chemical composition and antimicrobial activity of essential oil of thyme (Thymus vulgaris) from Eastern Morocco. Int. J. Agric. Biol. 2009, 11, 205–208. [Google Scholar]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W. Tocopherol-mediated synthesis of silver nanoparticles and preparation of antimicrobial PBAT/silver nanoparticles composite films. LWT-Food Sci. Technol. 2016, 72, 149–156. [Google Scholar] [CrossRef]
- Wang, L.F.; Rhim, J.W.; Hong, S.I. Preparation of poly (lactide)/poly (butylene adipate-co-terephthalate) blend films using a solvent casting method and their food packaging application. LWT-Food Sci. Technol. 2016, 68, 454–461. [Google Scholar] [CrossRef]
- Zhen, Z.; Ying, S.; Hongye, F.; Jie, R.; Tianbin, R. Preparation, characterization and properties of binary and ternary blends with thermoplastic starch, poly (lactic acid) and poly (butylene succinate). Polym. Renew. Resour. 2011, 2, 49–62. [Google Scholar] [CrossRef]
- Sharma, S.; Jaiswal, A.K.; Duffy, B.; Jaiswal, S. Ferulic acid incorporated active films based on poly (lactide)/poly (butylene adipate-co-terephthalate) blend for food packaging. Food Packag. Shelf Life 2020, 24, 100491. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W. Preparation of antibacterial poly (lactide)/poly (butylene adipate-co-terephthalate) composite films incorporated with grapefruit seed extract. Int. J. Biol. Macromol. 2018, 120, 846–852. [Google Scholar] [CrossRef] [PubMed]
- ASTM D882-18. Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- Petroudy, S.R.D. Physical and mechanical properties of natural fibers. In Advanced High Strength Natural Fibre Composites in Construction; Woodhead Publishing: Duxford, UK, 2017; pp. 59–83. [Google Scholar]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Innov. Food Sci. Emerg. Technol. 2016, 38, 231–237. [Google Scholar] [CrossRef]
- JIS, Z Japanese Standards Association. Antimicrobial Products–Test for Antimicrobial Activity and Afficacy; Minister of International Trade and Industry: Tokyo, Japan, 2008.
- Jaiswal, S.; Bhattacharya, K.; McHale, P.; Duffy, B. Dual effects of β-cyclodextrin-stabilised silver nanoparticles: Enhanced biofilm inhibition and reduced cytotoxicity. J. Mater. Sci. Mater. Med. 2015, 26, 52. [Google Scholar] [CrossRef] [Green Version]
- Pattanasiri, T.; Taparhudee, W.; Suppakul, P. Anaesthetic efficacy of clove oil-coated LDPE bag on improving water quality and survival in the Siamese fighting fish, β-splendens, during transportation. Aquac. Int. 2017, 25, 197–209. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ahmed, J.; Ejaz, M.; Mullah, M. Polylactide/graphene oxide nanosheets/clove essential oil composite films for potential food packaging applications. Int. J. Biol. Macromol. 2018, 107, 194–203. [Google Scholar] [CrossRef]
- Ejaz, M.; Arfat, Y.A.; Mulla, M.; Ahmed, J. Zinc oxide nanorods/clove essential oil incorporated Type B gelatin composite films and its applicability for shrimp packaging. Food Packag. Shelf Life 2018, 15, 113–121. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S.; Prodpran, T.; Sumpavapol, P.; Songtipya, P. Properties and antimicrobial activity of fish protein isolate/fish skin gelatin film containing basil leaf essential oil and zinc oxide nanoparticles. Food Hydrocoll. 2014, 41, 265–273. [Google Scholar] [CrossRef]
- Mulla, M.; Ahmed, J.; Al-Attar, H.; Castro-Aguirre, E.; Arfat, Y.A.; Auras, R. Antimicrobial efficacy of clove essential oil infused into chemically modified LLDPE film for chicken meat packaging. Food Control 2017, 73, 663–671. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Pires, C.; Ramos, C.; Batista, I.; Saraiva, J.A.; Nunes, M.L. Characterization of fish protein films incorporated with essential oils of clove, garlic and origanum: Physical, antioxidant and antibacterial properties. LWT-Food Sci. Technol. 2014, 59, 533–539. [Google Scholar] [CrossRef]
- Sanuja, S.; Agalya, A.; Umapathy, M. Studies on magnesium oxide reinforced chitosan bio-nanocomposite incorporated with clove oil for active food packaging application. Int. J. Polym. Mater. 2014, 63, 733–740. [Google Scholar] [CrossRef]
- Correa-Pacheco, Z.N.; Black-Solís, J.D.; Ortega-Gudiño, P.; Sabino-Gutiérrez, M.A.; Benítez-Jiménez, J.J.; Barajas-Cervantes, A.; Hurtado-Colmenares, L.B. Preparation and characterization of bio-based PLA/PBAT and cinnamon essential oil polymer fibers and life-cycle assessment from hydrolytic degradation. Polymers 2020, 12, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Medeiros, J.A.S.; Blick, A.P.; Galindo, M.V.; Alvim, I.D.; Yamashita, F.; Ueno, C.T.; Shirai, M.A.; Grosso, C.R.F.; Corradini, E.; Sakanaka, L.S. Incorporation of oregano essential oil microcapsules in Starch-Poly (Butylene Adipate Co-Terephthalate) (PBAT) Films. Macromol. Symp. 2019, 383, 1800052. [Google Scholar] [CrossRef] [Green Version]
- Salarbashi, D.; Tajik, S.; Ghasemlou, M.; Shojaee-Aliabadi, S.; Noghabi, M.S.; Khaksar, R. Characterization of soluble soybean polysaccharide film incorporated essential oil intended for food packaging. Carbohydr. Polym. 2013, 98, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Shojaee-Aliabadi, S.; Hosseini, H.; Mohammadifar, M.A.; Mohammadi, A.; Ghasemlou, M.; Ojagh, S.M.; Khaksar, R. Characterization of antioxidant-antimicrobial κ-carrageenan films containing Satureja hortensis essential oil. Int. J. Biol. Macromol. 2013, 52, 116–124. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of essential oils and homogenization conditions on properties of chitosan-based films. Food Hydrocoll. 2012, 26, 9–16. [Google Scholar] [CrossRef]
- Chen, C.; Xu, Z.; Ma, Y.; Liu, J.; Zhang, Q.; Tang, Z.; Xie, J. Properties, vapour-phase antimicrobial and antioxidant activities of active poly (vinyl alcohol) packaging films incorporated with clove oil. Food Control 2018, 88, 105–112. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Bio-based composite edible films containing Origanum vulgare L. essential oil. Ind. Crops Prod. 2015, 67, 403–413. [Google Scholar] [CrossRef]
- Yao, Z.C.; Chen, S.C.; Ahmad, Z.; Huang, J.; Chang, M.W.; Li, J.S. Essential oil bioactive fibrous membranes prepared via coaxial electrospinning. J. Food Sci. 2017, 82, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Vogler, E.A. Structure and reactivity of water at biomaterial surfaces. Adv. Colloid Interface Sci. 1998, 74, 69–117. [Google Scholar] [CrossRef]
- Song, N.B.; Lee, J.H.; Al Mijan, M.; Song, K.B. Development of a chicken feather protein film containing clove oil and its application in smoked salmon packaging. LWT-Food Sci. Technol. 2014, 57, 453–460. [Google Scholar] [CrossRef]
- Muppalla, S.R.; Kanatt, S.R.; Chawla, S.; Sharma, A. Carboxymethyl cellulose–polyvinyl alcohol films with clove oil for active packaging of ground chicken meat. Food Packag. Shelf Life 2014, 2, 51–58. [Google Scholar] [CrossRef]
- Khalil, A.A.; ur Rahman, U.; Khan, M.R.; Sahar, A.; Mehmood, T.; Khan, M. Essential oil eugenol: Sources, extraction techniques and nutraceutical perspectives. RSC Adv. 2017, 72, 32669–32681. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Bai, M.; Rashed, M.M.; Lin, L. The antibacterial activity of clove oil/chitosan nanoparticles embedded gelatin nanofibers against Escherichia coli O157: H7 biofilms on cucumber. Int. J. Food Microbiol. 2018, 266, 69–78. [Google Scholar] [CrossRef]




| Film | Lightness (L) | Redness, Greenness (a) | Yellowness, Blueness (b) | Total Colour Difference (∆E) | Transmittance (T280 (%)) | Transmittance (T600 (%)) |
|---|---|---|---|---|---|---|
| PLA/PBAT | 91.56 ± 0.36 a | −1.08 ± 0.02 c | 1.34 ± 0.03 a | 2.42 ± 0.35 d | 0.05 ± 0.00 b | 72.46 ± 0.63 a |
| PLA/PBAT-Thyme1% | 93.21 ± 0.08 b | −1.17 ± 0.02 b | 1.31 ± 0.04 a | 0.97 ±0.24 a | 0.04 ± 0.01 a | 73.43 ± 1.45 a |
| PLA/PBAT-Thyme5% | 92.93 ± 0.05 c | −1.27 ± 0.01 a | 1.69 ± 0.03 b | 1.22 ± 0.04 b | 0.04 ± 0.01 a | 81.28 ± 2.63 b |
| PLA/PBAT-Thyme10% | 92.95 ± 0.03 d | −1.30 ± 0.02 a | 1.89 ± 0.07 c | 1.29 ± 0.05 c | 0.04 ± 0.01 a | 81.28 ± 3.32 b |
| Film | Lightness (L) | Redness, Greenness (a) | Yellowness, Blueness (b) | Total Colour Difference ∆E | Transmittance (T280 (%)) | Transmittance (T600 (%)) |
|---|---|---|---|---|---|---|
| PLA/PBAT | 91.56 ± 0.54 a | −1.08 ± 0.02 d | 1.34 ± 0.03 a | 2.42 ± 0.03 b | 0.05 ± 0.00 c | 72.46 ± 1.07c |
| PLA/PBAT-Clove1% | 93.12 ± 0.96 a | −1.41 ± 0.01 c | 2.08 ± 0.05 b | 1.33 ± 0.04 a | 0.02 ± 0.00 b | 71.63 ± 0.35c |
| PLA/PBAT-Clove5% | 92.55 ± 0.79 a | −2.06 ± 0.03 b | 4.23 ± 0.25 c | 3.55 ± 0.04 c | 0.01 ± 0.00 a | 69.63 ± 0.68 b |
| PLA/PBAT-Clove10% | 92.14 ± 1.18 a | −2.99 ± 0.02 a | 7.78 ± 1.35 d | 7.15 ± 0.04 d | 0.01 ± 0.00 a | 65.53 ± 0.93a |
| Film | Thickness (µm) | Tensile Strength (TS (MPa)) | Elongation at Break (EB (%)) | Water Contact Angle (WCA (Degree)) |
|---|---|---|---|---|
| PLA/PBAT | 36.71 ± 4.16 a | 1.35 ± 0.03 b | 5.63 ± 0.41 b | 61.61 ± 2.82 a |
| PLA/PBAT-Thyme1% | 46.67 ± 5.73 a,b | 1.52 ± 0.08 b | 3.10 ± 0.38 a | 72.57 ± 1.54 b |
| PLA/PBAT–Thyme5% | 63.33 ± 10.17 b | 1.26 ± 1.26 b | 5.84 ± 0.25 b | 74.06 ± 3.00 b |
| PLA/PBAT–Thyme 10% | 113.33 ± 14.59 c | 0.96 ± 0.08 a | 16.51 ± 0.47 c | 80.57 ± 2.28 c |
| Film | Thickness (µm) | Tensile Strength (TS (MPa)) | Elongation at Break (EB (%)) | Water Contact Angle (WCA (Degree)) |
|---|---|---|---|---|
| PLA/PBAT | 36.71 ± 4.58 a | 1.35 ± 0.03 c | 5.63 ± 0.41 a | 61.61 ± 2.82 a |
| PLA/PBAT-Clove1% | 46.67 ± 3.59 a | 0.94 ± 0.08 b | 39.97 ± 0.09 d | 62.24 ± 5.75 a |
| PLA/PBAT-Clove5% | 73.33 ± 7.07 b | 0.89 ± 0.06 a, b | 27.58 ± 0.36 c | 64.69 ± 2.15 a |
| PLA/PBAT-Clove 10% | 106.67 ± 16.68 c | 0.79 ± 0.03 a | 25.67 ± 0.52 b | 74.74 ± 4.11 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Barkauskaite, S.; Duffy, B.; Jaiswal, A.K.; Jaiswal, S. Characterization and Antimicrobial Activity of Biodegradable Active Packaging Enriched with Clove and Thyme Essential Oil for Food Packaging Application. Foods 2020, 9, 1117. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9081117
Sharma S, Barkauskaite S, Duffy B, Jaiswal AK, Jaiswal S. Characterization and Antimicrobial Activity of Biodegradable Active Packaging Enriched with Clove and Thyme Essential Oil for Food Packaging Application. Foods. 2020; 9(8):1117. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9081117
Chicago/Turabian StyleSharma, Shubham, Sandra Barkauskaite, Brendan Duffy, Amit K. Jaiswal, and Swarna Jaiswal. 2020. "Characterization and Antimicrobial Activity of Biodegradable Active Packaging Enriched with Clove and Thyme Essential Oil for Food Packaging Application" Foods 9, no. 8: 1117. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9081117