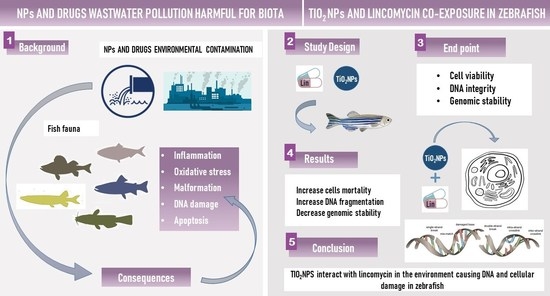
Evaluation of Zebrafish DNA Integrity after Individual and Combined Exposure to TiO2 Nanoparticles and Lincomycin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Design
2.3. Lincomycin Quantitation in Tank Water
2.4. Trypan Blue Staining
2.5. TUNEL Assay
2.6. Genomic DNA Isolation and the RAPD PCR Technique
2.7. Statistical Analysis
3. Results
3.1. Characterization and Analytical Determinations
3.2. Cell Viability
3.3. DNA Fragmentation
3.4. RAPD PCR Fingerprints
3.5. Genomic Template Stability (GTS%)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pretali, L.; Maraschi, F.; Cantalupi, A.; Albini, A.; Sturini, M. Water Depollution and Photo-Detoxification by Means of TiO2: Fluoroquinolone Antibiotics as a Case Study. Catalysts 2020, 10, 628. [Google Scholar] [CrossRef]
- Andreozzi, R.; Canterino, M.; Giudice, R.L.; Marotta, R.; Pinto, G.; Pollio, A. Lincomycin solar photodegradation, algal toxicity and removal from wastewaters by means of ozonation. Water Res. 2006, 40, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Bethesda, PubChem Compound Summary for CID 3000540, Lincomycin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Lincomycin (accessed on 16 February 2022).
- Spížek, J.; Řezanka, T. Lincomycin, clindamycin and their applications. Appl. Microbiol. Biotechnol. 2004, 64, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-H.; Kim, P.-G.; Park, J.-I. Pharmaceuticals in Environment and Their Implication in Environmental Health. Korean J. Environ. Health Sci. 2009, 35, 433–446. [Google Scholar] [CrossRef]
- Lissemore, L.; Hao, C.; Yang, P.; Sibley, P.; Mabury, S.; Solomon, K.R. An exposure assessment for selected pharmaceuticals within a watershed in Southern Ontario. Chemosphere 2006, 64, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Furlong, E.; Meyer, M.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999−2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [Green Version]
- Pomati, F.; Castiglioni, S.; Zuccato, E.; Fanelli, R.; Vigetti, D.; Rossetti, C.; Calamari, D. Effects of a Complex Mixture of Therapeutic Drugs at Environmental Levels on Human Embryonic Cells. Environ. Sci. Technol. 2006, 40, 2442–2447. [Google Scholar] [CrossRef]
- Wang, J.; Wei, H.; Zhou, X.; Li, K.; Wu, W.; Guo, M. Occurrence and risk assessment of antibiotics in the Xi’an section of the Weihe River, northwestern China. Mar. Pollut. Bull. 2019, 146, 794–800. [Google Scholar] [CrossRef]
- Sauvé, S.; Desrosiers, M. A review of what is an emerging contaminant. Chem. Cent. J. 2014, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Podlipná, R.; Skálová, L.; Seidlová, H.; Szotáková, B.; Kubíček, V.; Stuchlíková, L.R.; Jirásko, R.; Vaněk, T.; Vokřál, I. Biotransformation of benzimidazole anthelmintics in reed (Phragmites australis) as a potential tool for their detoxification in environment. Bioresour. Technol. 2013, 144, 216–224. [Google Scholar] [CrossRef]
- Vajda, A.M.; Barber, L.B.; Gray, J.L.; Lopez, E.M.; Bolden, A.M.; Schoenfuss, H.; Norris, D.O. Demasculinization of male fish by wastewater treatment plant effluent. Aquat. Toxicol. 2011, 103, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Batley, G.E.; Kirby, J.K.; McLaughlin, M.J. Fate and Risks of Nanomaterials in Aquatic and Terrestrial Environments. Acc. Chem. Res. 2013, 46, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Pavagadhi, S.; Sathishkumar, M.; Balasubramanian, R. Uptake of Ag and TiO2 nanoparticles by zebrafish embryos in the presence of other contaminants in the aquatic environment. Water Res. 2014, 55, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium Dioxide Nanoparticles in Food and Personal Care Products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Wang, L.; Wang, C.; Zhang, S.; Liu, H.; Li, S.; Wang, X. Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. J. Environ. Sci. 2019, 75, 40–53. [Google Scholar] [CrossRef]
- Grande, F.; Tucci, P. Titanium Dioxide Nanoparticles: A Risk for Human Health? Mini-Rev. Med. Chem. 2016, 16, 762–769. [Google Scholar] [CrossRef]
- Czajka, M.; Sawicki, K.; Sikorska, K.; Popek, S.; Kruszewski, M.; Kapka-Skrzypczak, L. Toxicity of titanium dioxide nanoparticles in central nervous system. Toxicol. In Vitro 2015, 29, 1042–1052. [Google Scholar] [CrossRef]
- Long, T.C.; Tajuba, J.; Sama, P.; Saleh, N.; Swartz, C.; Parker, J.; Hester, S.; Lowry, G.V.; Veronesi, B. Nanosize Titanium Dioxide Stimulates Reactive Oxygen Species in Brain Microglia and Damages Neurons in vitro. Environ. Health Perspect. 2007, 115, 1631–1637. [Google Scholar] [CrossRef] [Green Version]
- Trouiller, B.; Reliene, R.; Westbrook, A.; Solaimani, P.; Schiestl, R.H. Titanium Dioxide Nanoparticles Induce DNA Damage and Genetic Instability In vivo in Mice. Cancer Res. 2009, 69, 8784–8789. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.-S.; Baek, M.; Choi, S.-J. Comparative cytotoxicity of Al2O3, CeO2, TiO2 and ZnO nanoparticles to human lung cells. J. Nanosci. Nanotechnol. 2010, 10, 3453–3458. [Google Scholar] [CrossRef]
- Rocco, L.; Santonastaso, M.; Mottola, F.; Costagliola, D.; Suero, T.; Pacifico, S.; Stingo, V. Genotoxicity assessment of TiO2 nanoparticles in the teleost Danio rerio. Ecotoxicol. Environ. Saf. 2015, 113, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Tang, M. Toxicological study of metal and metal oxide nanoparticles in zebrafish. J. Appl. Toxicol. 2020, 40, 37–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; Husøy, T.; et al. Safety assessment of titanium dioxide (E171) as a food additive. EFSA J. 2021, 19, e06585. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Suresh, P.; Chandrasekaran, N.; Mukherjee, A. Antibiotic tetracycline enhanced the toxic potential of photo catalytically active P25 titanium dioxide nanoparticles towards freshwater algae Scenedesmus obliquus. Chemosphere 2021, 267, 128923. [Google Scholar] [CrossRef]
- Santonastaso, M.; Mottola, F.; Colacurci, N.; Iovine, C.; Pacifico, S.; Cammarota, M.; Cesaroni, F.; Rocco, L. In vitro genotoxic effects of titanium dioxide nanoparticles (n-TiO2) in human sperm cells. Mol. Reprod. Dev. 2019, 86, 1369–1377. [Google Scholar] [CrossRef]
- Santonastaso, M.; Mottola, F.; Iovine, C.; Cesaroni, F.; Colacurci, N.; Rocco, L. In Vitro Effects of Titanium Dioxide Nanoparticles (TiO2NPs) on Cadmium Chloride (CdCl2) Genotoxicity in Human Sperm Cells. Nanomaterials 2020, 10, 1118. [Google Scholar] [CrossRef]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Modeled Environmental Concentrations of Engineered Nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for Different Regions. Environ. Sci. Technol. 2009, 43, 9216–9222. [Google Scholar] [CrossRef]
- Hussain, S.; Joo, J.; Kang, J.; Kim, B.; Braun, G.B.; She, Z.-G.; Kim, D.; Mann, A.P.; Mölder, T.; Teesalu, T.; et al. Antibiotic-loaded nanoparticles targeted to the site of infection enhance antibacterial efficacy. Nat. Biomed. Eng. 2018, 2, 95–103. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Li, T.-J.; Tsai, B.-Y.; Chen, L.-K.; Lai, Y.-H.; Li, M.-J.; Tsai, C.-Y.; Tsai, P.-J.; Shieh, D.-B. Vancomycin-Loaded Nanoparticles Enhance Sporicidal and Antibacterial Efficacy for Clostridium difficile Infection. Front. Microbiol. 2019, 10, 1141. [Google Scholar] [CrossRef] [Green Version]
- Rocco, L.; Peluso, C.; Stingo, V. Micronucleus test and comet assay for the evaluation of zebrafish genomic damage induced by erythromycin and lincomycin. Environ. Toxicol. 2012, 27, 598–604. [Google Scholar] [CrossRef]
- Mottola, F.; Iovine, C.; Santonastaso, M.; Romeo, M.L.; Pacifico, S.; Cobellis, L.; Rocco, L. NPs-TiO2 and Lincomycin Coexposure Induces DNA Damage in Cultured Human Amniotic Cells. Nanomaterials 2019, 9, 1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horzmann, K.A.; Freeman, J.L. Making Waves: New Developments in Toxicology With the Zebrafish. Toxicol. Sci. 2018, 163, 5–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, H.-R.; Zhu, Y.-X.; Duan, Q.-Y.; Chen, Z.; Wu, F.-G. Nanomaterials meet zebrafish: Toxicity evaluation and drug delivery applications. J. Control. Release 2019, 311-312, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Nigro, M.; Bernardeschi, M.; Costagliola, D.; Della Torre, C.; Frenzilli, G.; Guidi, P.; Lucchesi, P.; Mottola, F.; Santonastaso, M.; Scarcelli, V.; et al. n-TiO2 and CdCl2 co-exposure to titanium dioxide nanoparticles and cadmium: Genomic, DNA and chromosomal damage evaluation in the marine fish European sea bass (Dicentrarchus labrax). Aquat. Toxicol. 2015, 168, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, A.K.; Reed, R.B.; Lee, S.; Bi, X.; Hanigan, D.; Yang, Y.; Ranville, J.F.; Herckes, P.; Westerhoff, P. Detection and Sizing of Ti-Containing Particles in Recreational Waters Using Single Particle ICP-MS. Bull. Environ. Contam. Toxicol. 2018, 100, 120–126. [Google Scholar] [CrossRef] [PubMed]
- EC–European Commission. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 276, 33–76. [Google Scholar]
- Mottola, F.; Scudiero, N.; Iovine, C.; Santonastaso, M.; Rocco, L. Protective activity of ellagic acid in counteract oxidative stress damage in zebrafish embryonic development. Ecotoxicol. Environ. Saf. 2020, 197, 110642. [Google Scholar] [CrossRef]
- Mottola, F.; Santonastaso, M.; Iovine, C.; Feola, V.; Pacifico, S.; Rocco, L. Adsorption of Cd to TiO2-NPs Forms Low Genotoxic Aggregates in Zebrafish Cells. Cells 2021, 10, 310. [Google Scholar] [CrossRef]
- Dantas, G.; Sommer, M.O.A.; Oluwasegun, R.D.; Church, G.M. Bacteria Subsisting on Antibiotics. Science 2008, 320, 100–103. [Google Scholar] [CrossRef] [Green Version]
- Dolliver, H.; Gupta, S. Antibiotic Losses in Leaching and Surface Runoff from Manure-Amended Agricultural Land. J. Environ. Qual. 2008, 37, 1227–1237. [Google Scholar] [CrossRef]
- Stoob, K.; Singer, H.P.; Mueller, S.R.; Schwarzenbach, R.P.; Stamm, C.H. Dissipation and Transport of Veterinary Sulfonamide Antibiotics after Manure Application to Grassland in a Small Catchment. Environ. Sci. Technol. 2007, 41, 7349–7355. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Qin, L.; Xie, C.; Liu, H.; Wang, Y.; Guan, C.; Huang, S. Distribution of antibiotics in alluvial sediment near animal breeding areas at the Jianghan Plain, Central China. Chemosphere 2017, 186, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Borgatta, M.; Waridel, P.; Decosterd, L.; Buclin, T.; Chèvre, N. Multigenerational effects of the anticancer drug tamoxifen and its metabolite 4-hydroxy-tamoxifen on Daphnia pulex. Sci. Total Environ. 2016, 545–546, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Gong, Y.; Liao, W.; Luo, Y.; Wu, C.; Wang, M.; Yang, Q. A review of cardiovascular toxicity of TiO2, ZnO and Ag nanoparticles (NPs). BioMetals 2018, 31, 457–476. [Google Scholar] [CrossRef]
- Mo, Y.; Zhang, Y.; Zhang, Q. Evaluation of pulmonary toxicity of nanoparticles by bronchoalveolar lavage. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2019; Volume 1894, pp. 313–322. [Google Scholar]
- Feng, X.; Chen, A.; Zhang, Y.; Wang, J.; Shao, L.; Wei, L. Central nervous system toxicity of metallic nanoparticles. Int. J. Nanomed. 2015, 10, 4321–4340. [Google Scholar]
- AshRrani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, L.; Ma, H.; Zhang, H.; Guo, L.-H. Quantitative Analysis of Reactive Oxygen Species Photogenerated on Metal Oxide Nanoparticles and Their Bacteria Toxicity: The Role of Superoxide Radicals. Environ. Sci. Technol. 2017, 51, 10137–10145. [Google Scholar] [CrossRef]
- Pawar, K.; Kaul, G. Toxicity of titanium oxide nanoparticles causes functionality and DNA damage in buffalo (Bubalus bubalis) sperm in vitro. Toxicol. Ind. Health 2014, 30, 520–533. [Google Scholar] [CrossRef]
- Meena, R.; Kajal, K.; R., P. Cytotoxic and Genotoxic Effects of Titanium Dioxide Nanoparticles in Testicular Cells of Male Wistar Rat. Appl. Biochem. Biotechnol. 2014, 175, 825–840. [Google Scholar] [CrossRef]
- Özgür, M.E.; Balcioglu, S.; Ulu, A.; Özcan, I.; Okumuş, F.; Köytepe, S.; Ateş, B. The in vitro toxicity analysis of titanium dioxide (TiO2) nanoparticles on kinematics and biochemical quality of rainbow trout sperm cells. Environ. Toxicol. Pharmacol. 2018, 62, 11–19. [Google Scholar] [CrossRef]
- Stenzel, M.H. The Trojan Horse Goes Wild: The Effect of Drug Loading on the Behavior of Nanoparticles. Angew. Chem. Int. Ed. 2021, 60, 2202–2206. [Google Scholar] [CrossRef] [PubMed]
- Wypij, M.; Świecimska, M.; Czarnecka, J.; Dahm, H.; Rai, M.; Golinska, P. Antimicrobial and cytotoxic activity of silver nanoparticles synthesized from two haloalkaliphilic actinobacterial strains alone and in combination with antibiotics. J. Appl. Microbiol. 2018, 124, 1411–1424. [Google Scholar] [CrossRef] [PubMed]
- Nowack, B.; Bucheli, T.D. Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007, 150, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Carrizales, M.; Velasco, K.I.; Castillo, C.; Flores, A.; Magaña, M.; Martinez-Castanon, G.A.; Martinez-Gutierrez, F. In Vitro Synergism of Silver Nanoparticles with Antibiotics as an Alternative Treatment in Multiresistant Uropathogens. Antibiotics 2018, 7, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohid, S.A.; Bhunia, A. Combining Antimicrobial Peptides with Nanotechnology: An Emerging Field in Theranostics. Curr. Protein Pept. Sci. 2020, 21, 413–428. [Google Scholar] [CrossRef]
- Jain, A.K.; Thareja, S. In vitro and in vivo characterization of pharmaceutical nanocarriers used for drug delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 524–539. [Google Scholar] [CrossRef] [Green Version]





| Substance Concentration | Exposure Days | Gained Bands (bp) | Lost Bands * (bp) |
|---|---|---|---|
| TiO2 NPs, 10 µg/L (n = 20) | 7 | 650 | 200, 300, 750 |
| 14 | 650, 850, 900 | 200, 300, 720, 750 | |
| Lincomycin, 100 mg/L (n = 20) | 7 | – | 200 |
| 14 | 350, 400 | 300, 320 | |
| TiO2 NPs, 10 µg/L + lincomycin, 100 mg/L (n = 20) | 7 | 350 | – |
| 14 | 350, 400 | 200, 320, 720, 750 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mottola, F.; Iovine, C.; Santonastaso, M.; Carfora, V.; Pacifico, S.; Rocco, L. Evaluation of Zebrafish DNA Integrity after Individual and Combined Exposure to TiO2 Nanoparticles and Lincomycin. Toxics 2022, 10, 132. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10030132
Mottola F, Iovine C, Santonastaso M, Carfora V, Pacifico S, Rocco L. Evaluation of Zebrafish DNA Integrity after Individual and Combined Exposure to TiO2 Nanoparticles and Lincomycin. Toxics. 2022; 10(3):132. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10030132
Chicago/Turabian StyleMottola, Filomena, Concetta Iovine, Marianna Santonastaso, Vincenzo Carfora, Severina Pacifico, and Lucia Rocco. 2022. "Evaluation of Zebrafish DNA Integrity after Individual and Combined Exposure to TiO2 Nanoparticles and Lincomycin" Toxics 10, no. 3: 132. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10030132