Potential Antagonistic Effects of Acrylamide Mitigation during Coffee Roasting on Furfuryl Alcohol, Furan and 5-Hydroxymethylfurfural
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analytical Methodology
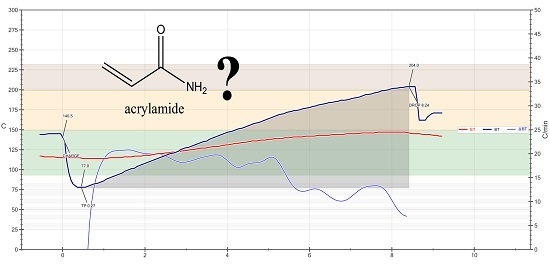
2.2. Samples and Roasting Experiments
2.3. Risk Assessment Methodology and Statistics
3. Results
3.1. Results of Roasting Experiments
3.2. Results of Commercial Sample Analyzes
3.3. Comparative Risk Assesment of Heat-Induced Contaminants in Coffee
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Sample ID | Sample Description | Category according to EU Regulation 2017/2158 | Year | AA (µg/kg) | FA (mg/kg) | Furan (mg/kg) | HMF (mg/kg) |
|---|---|---|---|---|---|---|---|
| 12119400 | Coffee substitute, soluble | Coffee substitutes from a mixture of cereals and chicory | 2012 | 803 | - | - | - |
| 12119400-1 | Coffee substitute, soluble | Coffee substitutes from a mixture of cereals and chicory | 2012 | 792 | - | - | - |
| 12119400-2 | Coffee substitute, soluble | Coffee substitutes from a mixture of cereals and chicory | 2012 | 806 | - | - | - |
| 12119400-3 | Coffee substitute, soluble | Coffee substitutes from a mixture of cereals and chicory | 2012 | 759 | - | - | - |
| 130122855 | Coffee substitute, soluble | Coffee substitutes from a mixture of cereals and chicory | 2013 | 664 | - | - | - |
| 130123291 | 100% soluble coffee | Instant (soluble) coffee | 2013 | 866 | - | - | - |
| 130123334 | Coffee, soluble | Instant (soluble) coffee | 2013 | 495 | - | - | - |
| 130124497 | Coffee substitute, soluble | Coffee substitutes exclusively from cereals | 2013 | 436 | - | - | - |
| 130124499 | Coffee, soluble | Instant (soluble) coffee | 2013 | 744 | - | - | - |
| 130127835 | 100% soluble coffee | Instant (soluble) coffee | 2013 | 796 | - | - | - |
| 130128813 | Coffee, soluble | Instant (soluble) coffee | 2013 | 325 | - | - | - |
| 130128818 | 100% soluble coffee | Instant (soluble) coffee | 2013 | 628 | - | - | - |
| 130130022 | Coffee substitute, soluble | Coffee substitutes from a mixture of cereals and chicory | 2013 | 591 | - | - | - |
| 130132127 | Coffee substitute, soluble | Coffee substitutes from a mixture of cereals and chicory | 2013 | 214 | - | - | - |
| 130132150 | Coffee substitute, soluble | Coffee substitutes exclusively from cereals | 2013 | 619 | - | - | - |
| 130237309 | Coffee substitute, soluble | Coffee substitutes from a mixture of cereals and chicory | 2013 | 387 | - | - | - |
| 150231135 | Coffee, soluble | Instant (soluble) coffee | 2015 | 1135 | - | - | - |
| 150231200 | Coffee, soluble | Instant (soluble) coffee | 2015 | 199 | - | - | - |
| 150231825 | Coffee substitute, soluble | Coffee substitutes exclusively from cereals | 2015 | 508 | - | - | - |
| 150231835 | Turkish coffee | Roast coffee | 2015 | 127 | - | - | - |
| 150309974 | Coffee | Roast coffee | 2015 | 70 | - | - | - |
| 150309977 | Coffee, decaffeinated | Instant (soluble) coffee | 2015 | 335 | - | - | - |
| 150334870 | Espresso Italiano | Roast coffee | 2015 | 132 | - | - | - |
| 150334875 | Coffee, soluble | Instant (soluble) coffee | 2015 | 356 | - | - | - |
| 150334880 | Coffee, soluble | Instant (soluble) coffee | 2015 | 320 | - | - | - |
| 150337674 | Coffee | Roast coffee | 2015 | 141 | - | - | - |
| 150337675 | Coffee, decaffeinated | Instant (soluble) coffee | 2015 | 585 | - | - | - |
| 150337676 | Coffee | Instant (soluble) coffee | 2015 | 452 | - | - | - |
| 160450717 | Malt coffee | Coffee substitutes exclusively from cereals | 2016 | 370 | - | - | - |
| 160451307 | Coffee, soluble | Instant (soluble) coffee | 2016 | 223 | - | - | - |
| 160451426 | Coffee, soluble | Instant (soluble) coffee | 2016 | 273 | - | - | - |
| 160451967 | Coffee substitute, soluble | Coffee substitutes from a mixture of cereals and chicory | 2016 | 361 | - | - | - |
| 160452173 | 100% soluble coffee, 100% Arabica | Instant (soluble) coffee | 2016 | 269 | - | - | - |
| 160452684 | Coffee substitute, soluble | Coffee substitutes exclusively from cereals | 2016 | 74 | - | - | - |
| 160454844 | Coffee, soluble | Instant (soluble) coffee | 2016 | 206 | - | - | - |
| 160472527 | Coffee substitute, soluble | Coffee substitutes from a mixture of cereals and chicory | 2016 | 407 | - | - | - |
| 160472529 | Coffee substitute, soluble | Coffee substitutes from a mixture of cereals and chicory | 2016 | 431 | - | - | - |
| 160509176 | Coffee, soluble | Instant (soluble) coffee | 2016 | 925 | - | - | - |
| 160509194 | Espresso | Roast coffee | 2016 | 447 | - | - | - |
| 160574673 | Coffee substitute, soluble | Coffee substitutes from a mixture of cereals and chicory | 2016 | 347 | - | - | - |
| 180352602 | Coffee substitute, soluble | Coffee substitutes from a mixture of cereals and chicory | 2018 | 650 | - | - | - |
| 180352683 | Coffee | Roast coffee | 2018 | 160 | 108 | 1.7 | 46 |
| 180365240 | Costa Rica Arabica coffee | Roast coffee | 2018 | 170 | 108 | 2.0 | 46 |
| 180379248 | India Monsooned | Roast coffee | 2018 | 150 | 74 | 5.4 | 34 |
| 180398113 | Coffee | Roast coffee | 2018 | 210 | 156 | 2.3 | 52 |
| 180409591 | Arabica-Robusta-mixture, coffee | Roast coffee | 2018 | 130 | 133 | 2.6 | 48 |
| 180420281 | coffee, organic | Roast coffee | 2018 | 110 | 122 | - | 57 |
| 180420519 | Coffee | Roast coffee | 2018 | 95 | 104 | 3.5 | 46 |
| 180433746 | Coffee | Roast coffee | 2018 | 310 | 76 | - | 53 |
| 180439193 | coffee, Ethiopia | Roast coffee | 2018 | 150 | - | - | - |
| 180444177 | Coffee | Roast coffee | 2018 | 170 | 121 | - | 42 |
| 180447473 | Coffee | Roast coffee | 2018 | 110 | 116 | - | 57 |
| 180453665 | Coffee Sumatra | Roast coffee | 2018 | 120 | 119 | - | 52 |
| 180468077 | Coffee | Roast coffee | 2018 | 130 | 122 | - | 51 |
| 180478363 | Coffee substitute, soluble | Instant (soluble) coffee | 2018 | 730 | - | - | - |
| 180481476 | Coffee substitute, soluble | Instant (soluble) coffee | 2018 | 110 | 64 | - | 40 |
| 180486743 | Coffee substitute, soluble | Instant (soluble) coffee | 2018 | 670 | - | - | - |
| 180486745 | Coffee substitute, soluble | Instant (soluble) coffee | 2018 | 460 | - | - | - |
| 180489109 | Coffee substitute, soluble | Instant (soluble) coffee | 2018 | 420 | - | - | - |
| 180489247 | Coffee substitute, soluble | Instant (soluble) coffee | 2018 | 440 | - | - | - |
| 180492032 | 100% soluble coffee, 100% Arabica | Instant (soluble) coffee | 2018 | 870 | - | - | - |
| 180492048 | Coffee, soluble | Instant (soluble) coffee | 2018 | 660 | - | - | - |
| 180494672 | Coffee, soluble | Instant (soluble) coffee | 2018 | 910 | - | - | - |
| 180504580 | Coffee, soluble | Instant (soluble) coffee | 2018 | 420 | - | - | - |
| 180520043 | 100% soluble coffee, 100% Arabica | Instant (soluble) coffee | 2018 | 690 | - | - | - |
| 180533580 | Pure Canephora coffee | Roast coffee | 2018 | 260 | 42 | - | 40 |
| 180533581 | Pure Canephora coffee | Roast coffee | 2018 | 270 | 37 | - | 49 |
| 180533582 | Arabica coffee | Roast coffee | 2018 | 270 | 78 | - | 46 |
| 180533583 | Arabica coffee | Roast coffee | 2018 | 460 | 55 | - | 43 |
| 180533584 | Arabica coffee | Roast coffee | 2018 | 330 | 60 | - | 35 |
| 180539910 | Coffee, soluble | Instant (soluble) coffee | 2018 | 600 | - | - | - |
| 180552699 | 100% Organic Arabica coffee | Roast coffee | 2018 | 120 | - | - | - |
| 180619399 | Coffee substitute, soluble | Coffee substitutes exclusively from cereals | 2018 | 400 | - | - | - |
| 180619831 | Coffee, soluble | Instant (soluble) coffee | 2018 | 240 | - | - | - |
| 180628887 | Espresso | Roast coffee | 2018 | 210 | - | - | - |
| 180631120 | Coffee substitute, soluble | Coffee substitutes from a mixture of cereals and chicory | 2018 | 460 | - | - | - |
| 180631416 | Coffee | Roast coffee | 2018 | 250 | - | - | - |
| 180638375 | Espresso | Roast coffee | 2018 | 100 | 99 | - | 38 |
| 180643299 | Coffee substitute, soluble | Coffee substitutes from a mixture of cereals and chicory | 2018 | 220 | - | - | - |
| 180643300 | Coffee substitute, soluble | Coffee substitutes from a mixture of cereals and chicory | 2018 | 1500 | - | - | - |
References
- Lachenmeier, D.W. Carcinogens in food: Opportunities and challenges for regulatory toxicology. Open Toxicol. J. 2009, 3, 30–34. [Google Scholar] [CrossRef]
- Wenzl, T.; Lachenmeier, D.W.; Gökmen, V. Analysis of heat-induced contaminants (acrylamide, chloropropanols and furan) in carbohydrate-rich food. Anal. Bioanal. Chem. 2007, 389, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W.; Przybylski, M.C.; Rehm, J. Comparative risk assessment of carcinogens in alcoholic beverages using the margin of exposure approach. Int. J. Cancer 2012, 131, E995–E1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pflaum, T.; Hausler, T.; Baumung, C.; Ackermann, S.; Kuballa, T.; Rehm, J.; Lachenmeier, D.W. Carcinogenic compounds in alcoholic beverages: An update. Arch. Toxicol. 2016, 90, 2349–2367. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Acrylamide. IARC Monogr. Eval. Carcinog. Risks Hum. 1994, 60, 389–433. [Google Scholar] [PubMed]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on acrylamide in food. EFSA J. 2015, 13, 4104. [Google Scholar] [CrossRef] [Green Version]
- Guenther, H.; Anklam, E.; Wenzl, T.; Stadler, R.H. Acrylamide in coffee: Review of progress in analysis, formation and level reduction. Food Addit. Contam. 2007, 24 (Suppl. 1), 60–70. [Google Scholar] [CrossRef] [PubMed]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Tornqvist, M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Tornqvist, M. Acrylamide: A cooking carcinogen? Chem. Res. Toxicol. 2000, 13, 517–522. [Google Scholar] [CrossRef]
- Deutscher Kaffeeverband. Jahresbericht 2004; Deutscher Kaffeeverband e.V.: Hamburg, Germany, 2005. [Google Scholar]
- Gutsche, B.; Weisshaar, R.; Buhlert, J. Acrylamide in food—Screening results from food control in Baden-Württemberg. Dtsch. Lebensm. Rundsch. 2002, 98, 437–443. [Google Scholar]
- Soares, C.M.D.; Alves, R.C.; Oliveira, M.B. Acrylamide in coffee: Influence of processing. In Processing and Impact on Active Components in Food; Preedy, V., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 575–582. [Google Scholar] [CrossRef]
- Weisshaar, R.; Gutsche, B. Formation of acrylamide in heated potato products—Model experiments pointing to asparagine as precursor. Dtsch. Lebensm. Rundsch. 2002, 98, 397–400. [Google Scholar]
- Bagdonaite, K.; Derler, K.; Murkovic, M. Determination of acrylamide during roasting of coffee. J. Agric. Food Chem. 2008, 56, 6081–6086. [Google Scholar] [CrossRef] [PubMed]
- Stadler, R.H.; Theurillat, V. Acrylamide in coffee. In Coffee: Emerging Health Effects and Disease Prevention; Chu, Y., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Soares, C.M.D.; Alves, R.C.; Oliveira, M.B. Factors affecting acrylamide levels in coffee beverages. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 217–224. [Google Scholar] [CrossRef]
- Lantz, I.; Ternite, R.; Wilkens, J.; Hoenicke, K.; Guenther, H.; van der Stegen, G.H. Studies on acrylamide levels in roasting, storage and brewing of coffee. Mol. Nutr. Food Res. 2006, 50, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Banchero, M.; Pellegrino, G.; Manna, L. Supercritical fluid extraction as a potential mitigation strategy for the reduction of acrylamide level in coffee. J. Food Eng. 2013, 115, 292–297. [Google Scholar] [CrossRef]
- Anese, M.; Nicoli, M.C.; Verardo, G.; Munari, M.; Mirolo, G.; Bortolomeazzi, R. Effect of vacuum roasting on acrylamide formation and reduction in coffee beans. Food Chem. 2014, 145, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Summa, C.A.; de la Calle, B.; Brohee, M.; Stadler, R.H.; Anklam, E. Impact of the roasting degree of coffee on the in vitro radical scavenging capacity and content of acrylamide. LWT Food Sci. Technol. 2007, 40, 1849–1854. [Google Scholar] [CrossRef]
- Senyuva, H.Z.; Gokmen, V. Study of acrylamide in coffee using an improved liquid chromatography mass spectrometry method: Investigation of colour changes and acrylamide formation in coffee during roasting. Food Addit. Contam. 2005, 22, 214–220. [Google Scholar] [CrossRef]
- Mojska, H.; Gielecinska, I. Studies of acrylamide level in coffee and coffee substitutes: Influence of raw material and manufacturing conditions. Roczniki Państwowego Zakładu Higieny 2013, 64, 173–181. [Google Scholar]
- Granby, K.; Fagt, S. Analysis of acrylamide in coffee and dietary exposure to acrylamide from coffee. Anal. Chim. Acta 2004, 520, 177–182. [Google Scholar] [CrossRef]
- Alves, R.C.; Soares, C.; Casal, S.; Fernandes, J.O.; Oliveira, M.B.P. Acrylamide in espresso coffee: Influence of species, roast degree and brew length. Food Chem. 2010, 119, 929–934. [Google Scholar] [CrossRef]
- Rexroth, A. Acrylamide. The new EU regulation 2017/2158. Dtsch. Lebensm. Rundsch. 2018, 114, 142–151. [Google Scholar]
- European Commission. Commission regulation (EU) 2017/2158 of 20 November 2017 establishing mitigation measures and benchmark levels for the reduction of the presence of acrylamide in food. Off. J. EU 2017, L304, 24–44. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Furan. IARC Monogr. Eval. Carcinog. Risks Hum. 1995, 63, 393–407. [Google Scholar] [PubMed]
- Grosse, Y.; Loomis, D.; Guyton, K.Z.; El, G.F.; Bouvard, V.; Benbrahim-Tallaa, L.; Mattock, H.; Straif, K. Some chemicals that cause tumours of the urinary tract in rodents. Lancet Oncol. 2017, 18, 1003–1004. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Furfuryl alcohol. IARC Monogr. Eval. Carcinog. Risks Hum. 2019, 119, 83–113. [Google Scholar]
- Monakhova, Y.B.; Lachenmeier, D.W. The Margin of exposure of 5-hydroxymethylfurfural (HMF) in alcoholic beverages. Environ. Health Toxicol. 2012, 27, e2012016. [Google Scholar] [CrossRef] [PubMed]
- NTP. NTP toxicology and carcinogenesis studies of 5-(hydroxymethyl)-2-furfural (CAS No. 67-47-0) in F344/N rats and B6C3F1 mice (gavage studies). Natl. Toxicol. Program Tech. Rep. Ser. 2010, 554, 1–180. [Google Scholar] [PubMed]
- EFSA. Update on furan levels in food from monitoring years 2004–2010 and exposure assessment. EFSA J. 2011, 9, 2347. [Google Scholar] [CrossRef]
- Lachenmeier, D.W. Furan in Coffee Products: A Probabilistic Exposure Estimation. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 887–893. [Google Scholar] [CrossRef]
- Morehouse, K.M.; Nyman, P.J.; Mcneal, T.P.; Dinovi, M.J.; Perfetti, G.A. Survey of furan in heat processed foods by headspace gas chromatography/mass spectrometry and estimated adult exposure. Food Addit. Contam. 2008, 25, 259–264. [Google Scholar] [CrossRef]
- Okaru, A.O.; Lachenmeier, D.W. The food and beverage occurrence of furfuryl alcohol and myrcene—Two emerging potential human carcinogens? Toxics 2017, 5, 9. [Google Scholar] [CrossRef]
- Okaru, A.O.; Lachenmeier, D.W. Processing Contaminants: Furfuryl Alcohol. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 543–549. [Google Scholar] [CrossRef]
- Albouchi, A.; Russ, J.; Murkovic, M. Parameters affecting the exposure to furfuryl alcohol from coffee. Food Chem. Toxicol. 2018, 118, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Albouchi, A.; Murkovic, M. Formation kinetics of furfuryl alcohol in a coffee model system. Food Chem. 2018, 243, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Arribas-Lorenzo, G.; Morales, F.J. Estimation of dietary intake of 5-hydroxymethylfurfural and related substances from coffee to Spanish population. Food Chem. Toxicol. 2010, 48, 644–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CEN/TC 275. EN 16618:2015. Food Analysis—Determination of Acrylamide in Food by Liquid Chromatography Tandem Mass Spectrometry (LC-ESI-MS/MS); European Committee for Standardization (CEN): Brussels, Belgium, 2015. [Google Scholar]
- Waizenegger, J.; Winkler, G.; Kuballa, T.; Ruge, W.; Kersting, M.; Alexy, U.; Lachenmeier, D.W. Analysis and risk assessment of furan in coffee products targeted to adolescents. Food Addit. Contam. Part A 2012, 29, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Monakhova, Y.B.; Ruge, W.; Kuballa, T.; Ilse, M.; Winkelmann, O.; Diehl, B.; Thomas, F.; Lachenmeier, D.W. Rapid approach to identify the presence of Arabica and Robusta species in coffee using 1H NMR spectroscopy. Food Chem. 2015, 182, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Mueller, U.; Agudo, A.; Carrington, C.; Doerge, D.; Hellenäs, K.E.; Leblanc, J.C.; Rao, M.; Renwick, A.; Slob, W.; Wu, Y. Acrylamide (Addendum). In WHO Food Additives Series 63. Safety Evaluation of Certain Contaminants in Food. Prepared by the Seventy-Second Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); WHO and FAO: Geneva, Switzerland, 2011; pp. 1–151. [Google Scholar]
- JECFA. Furfuryl alcohol and related substances. In Evaluation of Certain Food Additives/Seventy-Sixth Report of the Joint FAO/WHO Expert Committee on Food Additives (JECFA) (WHO Technical Report Series 974); World Health Organization: Geneva, Switzerland, 2012; pp. 67–69. [Google Scholar]
- Carthew, P.; DiNovi, M.; Setzer, R.W. Application of the margin of exposure (MoE) approach to substances in food that are genotoxic and carcinogenic: Example: Furan (CAS No. 110-00-9). Food Chem. Toxicol. 2010, 48, S69–S74. [Google Scholar] [CrossRef] [PubMed]
- ISO. ISO 6668:2008. Green Coffee—Preparation of Samples for Use in Sensory Analysis; International Organization for Standardization: Geneva, Switzerland, 2008. [Google Scholar]
- EFSA Scientific Committee. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA J. 2012, 10, 2579. [Google Scholar] [CrossRef] [Green Version]
- Bagdonaite, K.; Murkovic, M. Factors affecting the formation of acrylamide in coffee. Czech J. Food Sci. 2004, 22, 22–24. [Google Scholar] [CrossRef]
- Kocadagli, T.; Göncuöglu, N.; Hamzalioglu, A.; Gökmen, V. In depth study of acrylamide formation in coffee during roasting: Role of sucrose decomposition and lipid oxidation. Food Funct. 2012, 3, 970–975. [Google Scholar] [CrossRef]
- BfR. 5-HMF-Gehalte in Lebensmitteln Sind Nach Derzeitigem Wissenschaftlichen Kenntnisstand Gesundheitlich Unproblematisch. Stellungnahme No 030/2011 des BfR vom 15. Mai 2011 [According to the Current State of Scientific Knowledge 5-HMF Concentrations Occurring in Foods Do Not Give Rise to Safety Concerns. BfR Opinion Nr. 030/2011, 15 May 2011]; Bundesinstitut für Risikobewertung: Berlin, Germany, 2011. [Google Scholar]
- Codex Alimentarius. Code of Practice for the Reduction of Acrylamide in Foods (CAC/RCP 67-2009); Joint FAO/WHO Food Standards Programme: Rome, Italy, 2009. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Coffee, mate, and very hot beverages. IARC Monogr. Eval. Carcinog. Risks Hum. 2018, 116, 1–501. [Google Scholar]
- Loomis, D.; Guyton, K.Z.; Grosse, Y.; Lauby-Secretan, B.; El, G.F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K.; et al. Carcinogenicity of drinking coffee, mate, and very hot beverages. Lancet Oncol. 2016, 17, 877–878. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Coffee. IARC Monogr. Eval. Carcinog. Risks Hum. 1991, 51, 41–206. [Google Scholar] [PubMed]

| Profile | Charge 1 [°C] | Drop 2 [min] | Drop 2 [°C] | AUC 3 [°C·min] | Acrylamide [µg/kg] | Furfuryl Alcohol [mg/kg] | Furan [mg/kg] | HMF [mg/kg] |
|---|---|---|---|---|---|---|---|---|
| Scandinavian coffee | 145 | 08:22 | 200 | 555 | 470/480 | 70/93 | <1.2/2.5 | 40/47 |
| Coffee quick drying | 140 | 08:24 | 204 | 566 | 200/390 | 124/94 | 1.7/2.7 | 74/49 |
| Coffee slow drying | 85 | 10:21 | 205 | 673 | 210/420 | 128/92 | 1.5/2.6 | 62/43 |
| Espresso quick drying | 147 | 07:55 | 203 | 625 | 170/300 | 170/117 | 2.5/4.9 | 66/47 |
| Espresso slow drying | 140 | 10:48 | 207 | 762 | 150/290 | 173/133 | 2.6/5.0 | 78/42 |
| Neapolitan espresso | 145 | 10:06 | 222 | 796 | 130/250 | 223/189 | 3.6/7.6 | 84/32 |
| Category according to EU Regulation 2017/2158 | Year of Analysis | Number of Samples | Average [µg/kg] | Median [µg/kg] | 90th Percentile [µg/kg] |
|---|---|---|---|---|---|
| Roast coffee | 2002 (data from [11]) | 5 | 303 | 313 | 461 |
| Roast coffee | 2015 | 4 | 118 | 130 | 138 |
| Roast coffee | 2018 | 22 | 195 | 165 | 306 |
| Instant (soluble coffee) | 2013 | 6 | 642 | 686 | 831 |
| Instant (soluble coffee) | 2015 | 7 | 483 | 356 | 805 |
| Instant (soluble coffee) | 2016 | 5 | 379 | 269 | 664 |
| Instant (soluble coffee) | 2018 | 13 | 555 | 600 | 842 |
| Coffee substitutes exclusively from cereals | 2013–2018 | 6 | 401 | 418 | 563 |
| Coffee substitutes from a mixture of cereals and chicory | 2012–2018 | 16 | 587 | 525 | 805 |
| Contaminant | Average/P95 Content in Roasted Coffee | Average/P95 Exposure for Drinking 1 Cup of Coffee 1 | Toxicological Threshold 2 | Average/P95 Margin of Exposure (MOE) 3 |
|---|---|---|---|---|
| Acrylamide | 249/543 µg/kg [6] | 0.05/0.10 µg/kg bw/day | 0.18 mg/kg bw/day (BDML10) [43] | 3800/1700 |
| Furfuryl alcohol | 251/392 mg/kg [35] | 0.05/0.07 mg/kg bw/day | 53 mg/kg bw/day (NOEL) [44] | 1110/710 |
| HMF | 689/1688 mg/kg [39] | 0.13/0.32 mg/kg bw/day | 79 mg/kg bw/day (BMDL10) [30] | 600/250 |
| Furan | 38/107 µg/L [33] | 0.12/0.14 µg/kg bw/day [33] | 1.28 mg/kg bw/day (BMDL10) [45] | 42,134/3113 [33] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lachenmeier, D.W.; Schwarz, S.; Teipel, J.; Hegmanns, M.; Kuballa, T.; Walch, S.G.; Breitling-Utzmann, C.M. Potential Antagonistic Effects of Acrylamide Mitigation during Coffee Roasting on Furfuryl Alcohol, Furan and 5-Hydroxymethylfurfural. Toxics 2019, 7, 1. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics7010001
Lachenmeier DW, Schwarz S, Teipel J, Hegmanns M, Kuballa T, Walch SG, Breitling-Utzmann CM. Potential Antagonistic Effects of Acrylamide Mitigation during Coffee Roasting on Furfuryl Alcohol, Furan and 5-Hydroxymethylfurfural. Toxics. 2019; 7(1):1. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics7010001
Chicago/Turabian StyleLachenmeier, Dirk W., Steffen Schwarz, Jan Teipel, Maren Hegmanns, Thomas Kuballa, Stephan G. Walch, and Carmen M. Breitling-Utzmann. 2019. "Potential Antagonistic Effects of Acrylamide Mitigation during Coffee Roasting on Furfuryl Alcohol, Furan and 5-Hydroxymethylfurfural" Toxics 7, no. 1: 1. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics7010001