Mechanisms of Increased Indoxacarb Toxicity in Methoxyfenozide-Resistant Cotton Bollworm Helicoverpa armigera (Lepidoptera: Noctuidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. H. armigera Populations
2.2. Chemicals
2.3. Indoxacarb and Methoxyfenozide Toxicities to H. armigera
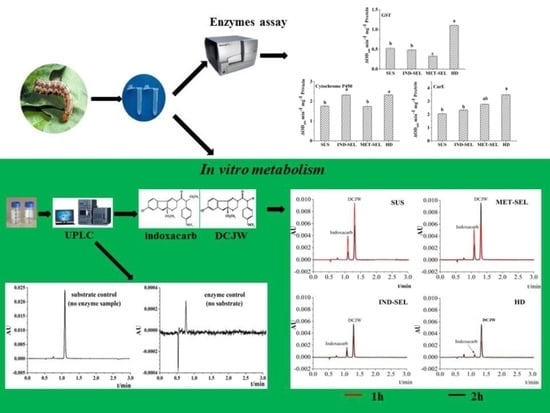
2.4. In Vitro Indoxacarb Metabolism
2.4.1. Crude-Enzyme Preparation from H. armigera Midgut
2.4.2. Indoxacarb N-Decarbomethoxylation Reaction
2.4.3. Ultraperformance Liquid Chromatography Analysis
2.4.4. DCJW Recovery
2.5. Enzymes and Protein-Concentration Assays
2.5.1. Carboxyl Esterase (CarE) Assay
2.5.2. GST Assay
2.5.3. Cytochrome P450 Assay
2.6. Statistical Analysis
3. Results
3.1. Susceptibility of H. armigera Field Populations to Indoxacarb and Methoxyfenozide
3.2. Toxicities of Indoxacarb and Methoxyfenozide against Laboratory Populations of H. armigera
3.3. UPLC Method of DCJW Validation
3.4. Comparison of N-Decarbomethoxylation Activity among Different H. armigera Populations
3.5. Detoxification-Enzyme Activities
4. Discussions
Author Contributions
Funding
Conflicts of Interest
References
- Fitt, G.P. The ecology of Heliothis species in relation to agroecosystems. Annu. Rev. Entomol. 1989, 34, 17–53. [Google Scholar]
- McCaffery, A.R.; King, A.B.S.; Walker, A.J.; El-Nayir, H. Resistance to synthetic pyrethroids in the bollworm Heliothis armigera from Andhra Pradesh, India. Pest. Manag. Sci. 1989, 27, 65–76. [Google Scholar]
- Cleary, A.J.; Cribb, B.W.; Murray, D.A.H. Helicoverpa armigera (Hübner): Can wheat stubble protect cotton plants against attack? Aust. J. Entomol. 2006, 45, 10–15. [Google Scholar]
- Mironidis, G.K.; Kapantaidaki, D.; Bentila, M.; Morou, E. Resurgence of the cotton bollworm Helicoverpa armigera in northern Greece associated with insecticide resistance. J. Insect Sci. 2013, 20, 505–512. [Google Scholar]
- Bertin, G.; Harder, H.H.; Riley, S.L.; Mccann, S.F.; Irving, S.N. DPX-MP062: A novel broad-spectrum insect control compound. Ann. ANPP 1997, 1, 185–191. [Google Scholar]
- Wing, K.D.; Schnee, M.E.; Sacher, M.; Connair, M.A. A novel oxadiazine insecticide is bioactivated in lepidopteran larvae. Arch. Insect Biochem. Physiol. 1998, 37, 91–103. [Google Scholar]
- Wing, K.D.; Sacher, M.; Kagaya, Y.; Tsurubuchi, Y.; Mulderig, L.; Connair, M.; Schnee, M. Bioactivation and mode of action of the oxadiazine indoxacarb in insects. Crop Prot. 2000, 19, 537–545. [Google Scholar]
- Song, W.Z.; Liu, Z.Q.; Dong, K. Molecular basis of differential sensitivity of insect sodium channels to DCJW, a bioactive metabolite of the oxadiazine insecticide indoxacarb. Neurotoxicology 2006, 27, 237–244. [Google Scholar]
- Li, F.G.; Ai, G.M.; Li, Y.S.; Zhu, C.Y.; Gao, X.W. Progress on mechanism of action and insecticide resistance of the oxadiazine indoxacarb in insects. Agrochemicals 2013, 8, 558–560. [Google Scholar]
- Gondhalekar, A.D.; Nakayasu, E.S.; Silva, I.; Cooper, B.; Scharf, M.E. Indoxacarb biotransformation in the German cockroach. Pestic. Biochem. Physiol. 2016, 134, 14–23. [Google Scholar]
- Tsurubuchi, Y.; Karasawa, A.; Nagata, K.T.; Shono, T.; Konno, Y. Insecticidal activity of oxadiazine insecticide indoxacarb and its N-decarbomethoxylated metabolite and their modulations of voltage-gated Na+ channels. Appl. Entomol. Zool. 2001, 36, 381–385. [Google Scholar]
- Zhao, X.L.; Ikeda, T.; Salgado, V.L.; Yeh, J.Z.; Narahashi, T. Block of two subtypes of sodium channels in cockroach neurons by indoxacarb insecticides. Neurotoxicology 2005, 26, 455–465. [Google Scholar] [PubMed]
- Shono, T.; Zhang, L.; Scott, J.G. Indoxacarb resistance in the housefly, Musca domestica. Pestic. Biochem. Physiol. 2004, 80, 106–112. [Google Scholar]
- Sayyed, A.H.; Wright, D.J. Genetics and evidence for an esterase-associated mechanism of resistance to indoxacarb in a field strain of diamondback moth (Lepidoptera: Plutellidae). Pest. Manag. Sci. 2006, 62, 1045–1051. [Google Scholar]
- Nehare, S.; Moharil, M.P.; Ghodki, B.S.; Lande, G.K.; Bisane, K.D.; Thakare, A.S.; Barkhade, U.P. Biochemical analysis and synergistic suppression of indoxacarb resistance in Plutella xylostella L. J. Asia Pac. Entomol. 2010, 13, 91–95. [Google Scholar]
- Wang, Q.Q.; Cui, L.; Wang, Q.Y.; Yang, H.Y.; Rui, C.H. Mechanisms of resistance to indoxacarb in Helicoverpa armigera (Lepidoptera: Noctuidae): Synergism of PBO, DEF and DEM and the activities of detoxification enzymes. Acta Entomol. Sin. 2017, 60, 912–919. [Google Scholar]
- Furlong, M.J.; Wright, D.J.; Dosdall, L.M. Diamondback moth ecology and management: Problems, progress and prospects. Annu. Rev. Entomol. 2013, 58, 517–541. [Google Scholar]
- Bird, L.J. Genetics, cross-resistance and synergism of indoxacarb resistance in Helicoverpa armigera (Lepidoptera: Noctuidae). Pest. Manag. Sci. 2017, 73, 575–581. [Google Scholar]
- Marak, R.M.; Firake, D.M.; Sontakke, P.P.; Behere, G.T. Mode of inheritance of indoxacarb resistance in diamondback moth, Plutella xylostella (L.) and cross resistance to different groups of pesticides. Phytoparasitica 2017, 2, 1–10. [Google Scholar]
- Ahmad, M.; Arif, M.I.; Ahmad, Z. Susceptibility of Helicoverpa armigera (Lepidoptera: Noctuidae) to new chemistries in Pakistan. Crop Prot. 2003, 22, 539–544. [Google Scholar]
- Zhang, S.Z.; Zhang, X.L.; Shen, J.; Li, D.Y.; Wan, H.; You, H.; Li, J.H. Cross-resistance and biochemical mechanisms of resistance to indoxacarb in the diamondback moth, Plutella xylostella. Pestic. Biochem. Physiol. 2017, 140, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Sayyed, A.H.; Saleem, M.A.; Ahmad, M. Evidence for field evolved resistance to newer insecticides in Spodoptera litura (lepidoptera: Noctuidae) from Pakistan. Crop Prot. 2008, 27, 1367–1372. [Google Scholar] [CrossRef]
- Wang, D.; Qiu, X.H.; Ren, X.X.; Zhang, W.C.; Wang, K.Y. Effects of spinosad on Helicoverpa armigera (Lepidoptera: Noctuidae) from China: Tolerance status, synergism and enzymatic responses. Pest Manag. Sci. 2009, 65, 1040–1046. [Google Scholar] [PubMed]
- Zhang, H.P.; Wang, Y.; Wang, R.F. HPLC determination of DCJW in rat Plasma and its application to pharmacokinetics studies. Chromatographia 2007, 66, 493–497. [Google Scholar]
- Pan, X.L.; Dong, F.S.; Chen, Z.L.; Xu, J.; Liu, X.G.; Wu, X.H.; Zheng, Y.Q. The application of chiral ultra-high-performance liquid chromatography tandem mass spectrometry to the separation of the zoxamide enantiomers and the study of enantioselective degradation process in agricultural plants. J. Chromatogr. A 2017, 1525, 87–95. [Google Scholar]
- Van Asperen, K. A study of housefly esterases by means of a sensitive colormetric method. J. Insect Physiol. 1962, 8, 401–414. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione-S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar]
- Oppenoorth, F.J.; Van der Pas, L.J.T.; Houx, N.W.H. Glutathione S-transferase and hydrolytic activity in a tetrachlorvinphos-resistant strain of housefly and their influence on resistance. Pestic. Biochem. Physiol. 1979, 11, 176–188. [Google Scholar]
- Cui, L.; Wang, Q.Q.; Qi, H.L.; Wang, Q.Y.; Yuan, H.Z.; Rui, C.H. Resistance selection of indoxacarb in Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae): Cross-resistance, biochemical mechanisms and associated fitness costs. Pest. Manag. Sci. 2018, 74, 2636–2644. [Google Scholar]
- Lai, T.C.; Li, J.; Su, J.Y. Monitoring of beet armyworm Spodoptera exigua, (Lepidoptera: Noctuidae) resistance to chlorantraniliprole in China. Pestic. Biochem. Physiol. 2011, 101, 198–205. [Google Scholar]
- Khambay, B.P.; Denholm, I.; Carlson, G.R.; Jacobson, R.M.; Dhadialla, T.S. Negative cross-resistance between dihydropyrazole insecticides and pyrethroids in houseflies, Musca domestica. Pest. Manag. Sci. 2001, 57, 761–763. [Google Scholar] [CrossRef]
- Cilek, J.E.; Dahlman, D.L.; Knapp, F.W. Possible mechanism of diazinon negative cross-resistance in pyrethroid-resistant horn flies (Diptera: Muscidae). J. Econ. Entomol. 1995, 88, 520–524. [Google Scholar] [CrossRef]
- Kolaczinski, J.H.; Curtis, C.F. Investigation of negative cross-resistance as a resistance-management tool for insecticide-treated nets. J. Econ. Entomol. 2004, 41, 930–934. [Google Scholar] [CrossRef] [Green Version]
- Georghiou, G.P. Genetics of resistance to insecticides in house flies and mosquitoes. Exp. Parasitol. 1969, 26, 224–255. [Google Scholar] [CrossRef]
- Elliott, M.; Famham, A.W.; Janes, N.F.; Johnson, D.M.; Pulman, D.A.; Sawicki, R.M. Insecticidal amides with selective potency against a resistant (super-kdr) strain of houseflies (Musca domestica L.). Agric. Biol. Chem. 1986, 50, 1347–1349. [Google Scholar] [CrossRef]
- Yamamoto, I.; Kyomura, N.; Takahashi, Y. Negatively correlated cross-resistance: Combinations of N-methylcarbamate with N-propylcarbamate or oxadiazolone for green rice leafhopper. Arch. Insect Biochem. Physiol. 1993, 22, 277–288. [Google Scholar] [CrossRef]
- Taylor, M.; Feyereisen, R. Molecular biology and evolution of resistance to toxicants. Mol. Biol. Evol. 1996, 13, 719–734. [Google Scholar] [CrossRef] [Green Version]
- Sheppard, C.D.; Joyce, J.A. Increased susceptibility of pyrethroid-resistant horn flies (Diptera: Muscidae) to chlorfenapyr. J. Econ. Entomol. 1998, 91, 398–400. [Google Scholar] [CrossRef]
- Hemingway, J.; Small, G.J.; Monro, A.G. Possible mechanisms of organophosphorus and carbamate insecticide resistance in German cockroaches (Dictyoptera, Blattellidae) from different geographical areas. J. Econ. Entomol. 1993, 86, 1623–1630. [Google Scholar] [CrossRef]
- Alves, A.P.; Allgeier, W.J.; Siegfried, B.D. Effects of the synergist S,S,S-tributyl phosphorotrithioate on indoxacarb toxicity and metabolism in the European corn borer, Ostrinia nubilalis (Hubner). Pestic. Biochem. Physiol. 2008, 90, 26–30. [Google Scholar] [CrossRef]
- Kao, C.H.; Sun, C.N. In vitro degradation of some organophosphorus insecticides by susceptible and resistant diamondback moth. Pestic. Biochem. Physiol. 1991, 41, 132–141. [Google Scholar] [CrossRef]
- Wang, X.L.; Su, W.; Zhang, J.H.; Yang, Y.H.; Dong, K.; Wu, Y.D. Two novel sodium channel mutations associated with resistance to indoxacarb and metaflumizone in the diamondback moth, Plutella xylostella. Insect Sci. 2016, 23, 50–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, M.; Hollingworth, D.R.M. Synergism of insecticides provides evidence of metabolic mechanisms of resistance in the obliquebanded leafroller Choristoneura rosaceana (Lepidoptera: Tortricidae). Pest. Manag. Sci. 2004, 60, 465–473. [Google Scholar] [CrossRef] [PubMed]




| Population | Insecticide | Slope (±SE) | LC50 (95% CL) (μg mL−1) | N a | Indoxacarb RR b | Methoxyfenozide RR c |
|---|---|---|---|---|---|---|
| SUS | Indoxacarb | 1.52 ± 0.092 | 8.12 (6.70–9.80) | 210 | 1.83 | |
| Methoxyfenozide | 1.11 ± 0.105 | 83.73 (69.19–101.32) | 210 | 2.81 | ||
| IND-SEL | Indoxacarb | 1.52 ± 0.020 | 35.42 (34.23–36.65) | 210 | 8.00 | |
| Methoxyfenozide | 1.74 ± 0.097 | 29.79 (25.99–34.15) | 210 | 1.00 | ||
| MET-SEL | Indoxacarb | 1.05 ± 0.054 | 4.43 (3.63–5.41) | 210 | 1.00 | |
| Methoxyfenozide | 1.08 ± 0.075 | 408.04 (356.03–467.66) | 210 | 13.70 |
| Applied (μg L−1) | Found (μg L−1) | Recovery Ratio (%) ± SE | RSD (%) |
|---|---|---|---|
| 40 | 39.1 | 98.39 ± 0.89 a | 1.55 |
| 200 | 181.6 | 91.82 ± 1.90 b | 3.30 |
| 1000 | 915.6 | 91.56 ± 0.56 b | 0.97 |
| H. armigera Population | Concentration of DCJW (μg L−1) | ||
|---|---|---|---|
| N a | 1 h | 2 h | |
| SUS | 30 | 353.45 ± 17.07 a | 224.01± 7.32 b |
| MET-SEL | 30 | 442.36 ± 57.94 a | 474.41± 26.54 a |
| IND-SEL | 30 | 207.28 ± 9.35 b | 263.33 ± 21.37 b |
| HD | 30 | 97.30 ± 9.04 b | 150.39 ± 23.62 b |
| H. armigera Population | N-Decarbomethoxylation Activity (pmol (DCJW) mg−1 protein min−1) (Mean ± SE) | ||||
|---|---|---|---|---|---|
| N a | 1 h | Activity Ratio | 2 h | Activity Ratio | |
| SUS | 30 | 7.39 ± 0.21 a | 4.59 | 2.13 ± 0.31 b | 0.86 |
| MET-SEL | 30 | 6.94 ± 0.08 a | 4.31 | 4.19 ± 0.23 a | 1.68 |
| IND-SEL | 30 | 3.14 ± 0.42 b | 1.95 | 1.98 ± 0.11 b | 0.79 |
| HD | 30 | 1.61 ± 0.13 b | 1 | 2.49 ± 0.40 b | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Rui, C.; Wang, Q.; Wang, L.; Li, F.; Nahiyoon, S.A.; Yuan, H.; Cui, L. Mechanisms of Increased Indoxacarb Toxicity in Methoxyfenozide-Resistant Cotton Bollworm Helicoverpa armigera (Lepidoptera: Noctuidae). Toxics 2020, 8, 71. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics8030071
Wang Q, Rui C, Wang Q, Wang L, Li F, Nahiyoon SA, Yuan H, Cui L. Mechanisms of Increased Indoxacarb Toxicity in Methoxyfenozide-Resistant Cotton Bollworm Helicoverpa armigera (Lepidoptera: Noctuidae). Toxics. 2020; 8(3):71. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics8030071
Chicago/Turabian StyleWang, Qinqin, Changhui Rui, Qiyuan Wang, Li Wang, Fugen Li, Shahzad Ali Nahiyoon, Huizhu Yuan, and Li Cui. 2020. "Mechanisms of Increased Indoxacarb Toxicity in Methoxyfenozide-Resistant Cotton Bollworm Helicoverpa armigera (Lepidoptera: Noctuidae)" Toxics 8, no. 3: 71. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics8030071