Biosynthesized Iron Oxide Nanoparticles (Fe3O4 NPs) Mitigate Arsenic Toxicity in Rice Seedlings
Abstract
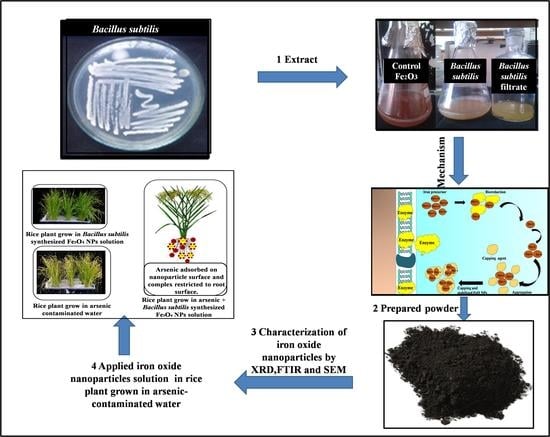
:1. Introduction
2. Materials and Methods
2.1. Experimental Methods
2.2. Application of Bacillus subtilis-Synthesized Fe3O4 NPs on Seed Germination
2.3. Determination of Physiological Parameters
2.4. Experimental Design and Statistical Analysis
3. Results
3.1. Fe3O4 NPs Synthesis and Characterization
3.2. Effect of Fe3O4 NPs on Seeds Germination and Growth of Seedling against Arsenic Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zubair, M.; Martyniuk, C.J.; Shaheen, A. Rising level of arsenic in water and fodder: A growing threat to livestock and human populations in Pakistan. Toxin Rev. 2018, 37, 171–181. [Google Scholar] [CrossRef]
- Shahid, M.; Khalid, M.; Dumat, C.; Khalid, S.; Niazi, N.K.; Imran, M.; Bibi, I.; Ahmad, I.; Hammad, H.M.; Tabassum, R.A. Arsenic level and risk assessment of groundwater in Vehari, Punjab Province, Pakistan. Expos. Health 2018, 10, 229–239. [Google Scholar] [CrossRef]
- Li, R.; Kuo, Y.M.; Liu, W.W.; Jang, C.S.; Zhao, E.; Yao, L. Potential health risk assessment through ingestion and dermal contact arsenic-contaminated groundwater in Jianghan Plain, China. Environ. Geochem. Health 2018, 40, 1585–1599. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Banerjee, N.; Bhattacharjee, P.; Mondal, D.; Lythgoe, P.R.; Martinez, M.; Pan, J.; Polya, D.A.; Giri, A.K. High arsenic in rice is associated with elevated genotoxic effects in humans. Sci. Rep. 2013, 3, 2195. [Google Scholar] [CrossRef] [Green Version]
- Lim, K.T.; Shukor, M.Y.; Wasoh, H. Physical, chemical, and biological methods for the removal of arsenic compounds. Biomed. Res. Int. 2014, 503784. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, P.A.; Augustine, R.; Kannan, M. Extracellular biosynthesis of iron oxide nanoparticles by Bacillus subtilis strains isolated from rhizosphere soil. Biotechnol. Bioproc. Eng. 2012, 17, 835–840. [Google Scholar] [CrossRef]
- Casentini, B.; Gallo, M.; Baldi, F. Arsenate and arsenite removal from contaminated water by iron oxides nanoparticles formed inside a bacterial exopolysaccharide. J. Environ. Chem. Eng. 2019, 7, 102908. [Google Scholar] [CrossRef] [Green Version]
- Saif, S.; Tahir, A.; Chen, Y. Green synthesis of iron nanoparticles and their environmental applications and implications. Nanomaterials 2016, 6, 209. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, F.; Ashraf, N.; Ashraf, T.; Zhou, R.B.; Yin, D.C. Biological synthesis of metallic nanoparticles (MNPs) by plants and microbes: Their cellular uptake, biocompatibility, and biomedical applications. Appl. Microbiol. Biot. 2019, 103, 2913–2935. [Google Scholar] [CrossRef]
- Praveen, A.; Khan, E.; Perwez, M.; Sardar, M.; Gupta, M. Iron oxide nanoparticles as nano-adsorbents: A possible way to reduce arsenic phytotoxicity in Indian mustard plant (Brassica juncea L.). J. Plant Growth Regul. 2018, 37, 612–624. [Google Scholar] [CrossRef]
- Srivastava, G.; Das, C.K.; Das, A.; Singh, S.K.; Roy, M.; Kim, H.; Sethy, N.; Kumar, A.; Sharma, R.K.; Singh, S.K.; et al. Seed treatment with iron pyrite (FeS2) nanoparticles increases the production of spinach. RSC Adv. 2014, 4, 58495–58504. [Google Scholar] [CrossRef]
- Ejaz, M.; Raja, N.I.; Mashwani, Z.R.; Ahmad, M.S.; Hussain, M.; Iqbal, M. Effect of silver nanoparticles and silver nitrate on growth of rice under biotic stress. IET Nanobiotechnol. 2018, 12, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Asif, S.; Ilyas, N.; Raja, N.I.; Hussain, M.; Shabir, S.; Faz MN, A.; Rauf, A. Effect of Plant Derived Smoke on Germination and Post Germination Expression of Wheat. Am. J. Plant Sci. 2016, 7, 806–813. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, M.K.; Ahmed, S.H.; Hameed, R.S.; Alkharkhi, I.H.T. Synthesis characterization of new nanoparticles derived from iron oxide and Beta vulgaris extracts and its bioactivity against Pantoea spp. Indian J. Med. Forensic. Med. Toxicol. 2019, 13, 362–366. [Google Scholar] [CrossRef]
- Tiquia-Arashiro, S.M.; Rodrigues, D.F. Chapter 1. In Extremophiles: Applicationsin in Nanotechnology; Springer International Publishing: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Rawat, M.; Nayan, R.; Negi, B.; Zaidi MG, H.; Arora, S. Physio-biochemical basis of iron-sulfide nanoparticle induced growth and seed yield enhancement in B. juncea. Plant Physiol. Biochem. 2017, 118, 274–284. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, Z.T.; Rui, Y.K.; Ren, J.Y.; Hou, T.Q.; Wu, S.J.; Liu, L.M. Effect of Different Nanoparticles on Seed Germination and Seedling Growth in Rice. In Proceedings of the 2nd Annual International Conference on Advanced Material Engineering, Wuhan, China, 15–17 April 2016; pp. 166–173. [Google Scholar]
- Pandey, C.; Khan, E.; Panthri, M.; Tripathi, R.D.; Gupta, M. Impact of silicon on Indian mustard (Brassica juncea L.) root traits by regulating growth parameters, cellular antioxidants and stress modulators under arsenic stress. Plant Physiol. Biochem. 2016, 104, 216–225. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahman, M.A.; Maki, T.; Hasegawa, H. Phytotoxicity of arsenate and salinity on early seedling growth of rice (oryza sativa l.): A threat to sustainable rice cultivation in South and South-East Asia. Bull. Environ. Contam. Tox. 2012, 88, 695–702. [Google Scholar] [CrossRef] [Green Version]
- Raju, D.; Mehta, U.J.; Beedu, S.R. Biogenic green synthesis of monodispersed gum kondagogu (Cochlospermumgossypium) iron nanocomposite material and its application in germination and growth of mung bean (Vignaradiata) as a plant model. IET Nanobiotechnol. 2016, 10, 141–146. [Google Scholar] [CrossRef]
- Gujjar, R.S.; Akhtar, M.; Singh, M. Transcription factors in abiotic stress tolerance. Indian J. Plant Physiol. 2014, 19, 306–316. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Yanful, E.K.; Pratt, A.R. Arsenic removal from aqueous solutions by mixed magnetite-maghemite nanoparticles. Environ. Earth Sci. 2011, 64, 411–423. [Google Scholar] [CrossRef]
- Kalita, J.; Pradhan, A.K.; Shandilya, Z.M.; Tanti, B. Arsenic Stress Responses and Tolerance in Rice: Physiological, Cellular and Molecular Approaches. Rice Sci. 2018, 25, 235–249. [Google Scholar] [CrossRef]
- Karimi, N.; Shayesteh, L.S.; Ghasempour, H. Effect of arsenic on germination of IsatiscappadocicaDesv., a newly discovered arsenic hyperaccumulator. Acta Biol. Cracov. Bot. 2013, 55, 61–67. [Google Scholar]
- Du, L.; Xia, X.; Lan, X.; Liu, M.; Zhao, L.; Zhang, P.; Wu, Y. Influence of arsenic stress on physiological, biochemical, and morphological characteristics in seedlings of two cultivars of maize (Zea mays L.). Water Air Soil Pollut. 2017, 228, 55. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, H.; Lal, R. Effects of Stabilized Nanoparticles of Copper, Zinc, Manganese, and Iron Oxides in Low Concentrations on Lettuce (Lactuca sativa) Seed Germination: Nanotoxicants or Nanonutrients. Water Air Soil Poll. 2016, 227, 42. [Google Scholar] [CrossRef]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 8263. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhou, Z.; Zhang, Y.; Xie, X.; Li, Y.; Shen, X. Uptake and Accumulation Characteristics of Arsenic and Iron Plaque in Rice at Different Growth Stages. Commun. Soil. Sci. Plan. 2015, 46, 2509–2522. [Google Scholar] [CrossRef]
- Ngo, Q.B.; Dao, T.H.; Nguyen, H.C.; Tran, X.T.; Van Nguyen, T.; Khuu, T.D.; Huynh, T.H. Effects of nanocrystalline powders (Fe, Co and Cu) on the germination, growth, crop yield and product quality of soybean (Vietnamese species DT-51). Adv. Nat. Sci. Nanosci. 2014, 5, 015016. [Google Scholar] [CrossRef]
- Ndeh, N.T.; Maensiri, S.; Maensiri, D. The effect of green synthesized gold nanoparticles on rice germination and roots. Adv. Nat. Sci. Nanosci. 2017, 8, 035008. [Google Scholar] [CrossRef] [Green Version]
- Kasote, D.M.; Lee, J.H.; Jayaprakasha, G.K.; Patil, B.S. Seed Priming with Iron Oxide Nanoparticles Modulate Antioxidant Potential and Defense-Linked Hormones in Watermelon Seedlings. ACS Sustain. Chem. Eng. 2019, 7, 5142–5151. [Google Scholar] [CrossRef]
- Li, J.; Chang, P.R.; Huang, J.; Wang, Y.; Yuan, H.; Ren, H. Physiological effects of magnetic iron oxide nanoparticles towards watermelon. J. Nanosci. Nanotechnol. 2013, 13, 5561–5567. [Google Scholar] [CrossRef]
- Iftikhar, A.; Ali, S.; Yasmeen, T.; Arif, M.S.; Zubair, M.; Rizwan, M.; Soliman, M.H. Effect of gibberellic acid on growth, photosynthesis and antioxidant defense system of wheat under zinc oxide nanoparticle stress. Environ. Pollut. 2019, 254 Pt B, 113109. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.; Akhtar, N.; Rehman, S.U.; Shujah, S.; Rha, E.S.; Jamil, M. Biosynthesized Iron Oxide Nanoparticles (Fe3O4 NPs) Mitigate Arsenic Toxicity in Rice Seedlings. Toxics 2021, 9, 2. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics9010002
Khan S, Akhtar N, Rehman SU, Shujah S, Rha ES, Jamil M. Biosynthesized Iron Oxide Nanoparticles (Fe3O4 NPs) Mitigate Arsenic Toxicity in Rice Seedlings. Toxics. 2021; 9(1):2. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics9010002
Chicago/Turabian StyleKhan, Sehresh, Nazneen Akhtar, Shafiq Ur Rehman, Shaukat Shujah, Eui Shik Rha, and Muhammad Jamil. 2021. "Biosynthesized Iron Oxide Nanoparticles (Fe3O4 NPs) Mitigate Arsenic Toxicity in Rice Seedlings" Toxics 9, no. 1: 2. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics9010002