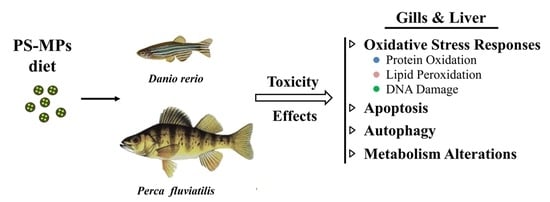
Toxicity and Functional Tissue Responses of Two Freshwater Fish after Exposure to Polystyrene Microplastics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Polystyrene Microplastics (PS-MPs)
2.2. Characterization of Polystyrene Microplastics (PS-MPs) in Fish Parts
2.3. Fish Husbandry
2.4. Ethical Statement
2.5. Food Preparation
2.6. EC50 Estimation (1st Experimental Design)
2.7. Fish Feeding Exposure to PS-MPs (2nd Experimental Design)
2.8. Tissue Sampling
2.9. Molecular and Biochemical Analyses
2.10. Metabolomics
2.10.1. Sample Preparation
2.10.2. LC-MS/MS Analysis
2.10.3. Data Analysis and Interpretation
2.11. Statistical Analyses
3. Results
3.1. PS-MPs Characterization
3.2. Molecular and Biochemical Responses
3.2.1. Oxidative Stress Biomarkers
3.2.2. Apoptosis and Autophagy
3.3. Inter-Species and Inter-Tissue Comparisons of Molecular and Biochemical Parameters
3.4. Correlation between Biochemical Indicators
3.5. Metabolomics
4. Discussion
4.1. Oxidative Stress
4.2. Molecular Events, Apoptosis and Autophagy
4.3. Metabolomics
4.4. Interspecies and Intertissue Comparisons
4.5. Internalization-Toxicity Induction by PS-MPs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setälä, O.; Fleming-Lehtinen, V.; Lehtiniemi, M. Ingestion and transfer of microplastics in the planktonic food web. Environ. Pollut. 2014, 185, 77–83. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic Ingestion by Zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef]
- Mattsson, K.; Ekvall, M.T.; Hansson, L.-A.; Linse, S.; Malmendal, A.; Cedervall, T. Altered Behavior, Physiology, and Metabolism in Fish Exposed to Polystyrene Nanoparticles. Environ. Sci. Technol. 2015, 49, 553–561. [Google Scholar] [CrossRef]
- Della Torre, C.; Bergami, E.; Salvati, A.; Faleri, C.; Cirino, P.; Dawson, K.A.; Corsi, I. Accumulation and Embryotoxicity of Polystyrene Nanoparticles at Early Stage of Development of Sea Urchin Embryos Paracentrotus lividus. Environ. Sci. Technol. 2014, 48, 12302–12311. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [Green Version]
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A Global Perspective on Microplastics. J. Geophys. Res. Oceans 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Ricciardi, M.; Pironti, C.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Aquatic Environment: Occurrence, Persistence, Analysis, and Human Exposure. Water 2021, 13, 973. [Google Scholar] [CrossRef]
- Siegfried, M.; Koelmans, A.A.; Besseling, E.; Kroeze, C. Export of microplastics from land to sea. A modelling approach. Water Res. 2017, 127, 249–257. [Google Scholar] [CrossRef]
- Napper, I.E.; Baroth, A.; Barrett, A.C.; Bhola, S.; Chowdhury, G.W.; Davies, B.F.R.; Duncan, E.M.; Kumar, S.; Nelms, S.E.; Hasan Niloy, M.N.; et al. The abundance and characteristics of microplastics in surface water in the transboundary Ganges River. Environ. Pollut. 2021, 274, 116348. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Kelly, F.J.; Wright, S.L. Advances and challenges of microplastic pollution in freshwater ecosystems: A UK perspective. Environ. Pollut. 2020, 256, 113445. [Google Scholar] [CrossRef]
- Zhou, R.; Lu, G.; Yan, Z.; Jiang, R.; Bao, X.; Lu, P. A review of the influences of microplastics on toxicity and transgenerational effects of pharmaceutical and personal care products in aquatic environment. Sci. Total Environ. 2020, 732, 139222. [Google Scholar] [CrossRef] [PubMed]
- Enyoh, C.E.; Shafea, L.; Verla, A.W.; Verla, E.N.; Qingyue, W.; Chowdhury, T.; Paredes, M. Microplastics Exposure Routes and Toxicity Studies to Ecosystems: An Overview. Environ. Anal. Health Toxicol. 2020, 35, e2020004. [Google Scholar] [CrossRef] [Green Version]
- Araújo, A.P.d.C.; Rocha, T.L.; e Silva, D.d.M.; Malafaia, G. Micro(nano)plastics as an emerging risk factor to the health of amphibian: A scientometric and systematic review. Chemosphere 2021, 283, 131090. [Google Scholar] [CrossRef]
- Canniff, P.M.; Hoang, T.C. Microplastic ingestion by Daphnia magna and its enhancement on algal growth. Sci. Total Environ. 2018, 633, 500–507. [Google Scholar] [CrossRef] [Green Version]
- Caron, A.G.M.; Thomas, C.R.; Berry, K.L.E.; Motti, C.A.; Ariel, E.; Brodie, J.E. Ingestion of microplastic debris by green sea turtles (Chelonia mydas) in the Great Barrier Reef: Validation of a sequential extraction protocol. Mar. Pollut. Bull. 2018, 127, 743–751. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, H.; Chen, B.; Sun, X.; Qu, K.; Xia, B. Microplastic ingestion in deep-sea fish from the South China Sea. Sci. Total Environ. 2019, 677, 493–501. [Google Scholar] [CrossRef]
- Redondo-Hasselerharm, P.E.; Falahudin, D.; Peeters, E.T.H.M.; Koelmans, A.A. Microplastic Effect Thresholds for Freshwater Benthic Macroinvertebrates. Environ. Sci. Technol. 2018, 52, 2278–2286. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.; Ryan, P.G. Micro-plastic ingestion by waterbirds from contaminated wetlands in South Africa. Mar. Pollut. Bull. 2018, 126, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Nelms, S.E.; Barnett, J.; Brownlow, A.; Davison, N.J.; Deaville, R.; Galloway, T.S.; Lindeque, P.K.; Santillo, D.; Godley, B.J. Microplastics in marine mammals stranded around the British coast: Ubiquitous but transitory? Sci. Rep. 2019, 9, 1075. [Google Scholar] [CrossRef] [Green Version]
- Rotjan, R.D.; Sharp, K.H.; Gauthier, A.E.; Yelton, R.; Lopez, E.M.B.; Carilli, J.; Kagan, J.C.; Urban-Rich, J. Patterns, dynamics and consequences of microplastic ingestion by the temperate coral, Astrangia poculata. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190726. [Google Scholar] [CrossRef] [Green Version]
- Scherer, C.; Weber, A.; Lambert, S.; Wagner, M. Interactions of Microplastics with Freshwater Biota. In Freshwater Microplastics: Emerging Environmental Contaminants? Wagner, M., Lambert, S., Eds.; Springer International Publishing: Cham, Switzeland, 2018; pp. 153–180. [Google Scholar]
- Triebskorn, R.; Braunbeck, T.; Grummt, T.; Hanslik, L.; Huppertsberg, S.; Jekel, M.; Knepper, T.P.; Krais, S.; Müller, Y.K.; Pittroff, M.; et al. Relevance of nano- and microplastics for freshwater ecosystems: A critical review. TrAC Trends Anal. Chem. 2019, 110, 375–392. [Google Scholar] [CrossRef]
- Xiao, Y.; Jiang, X.; Liao, Y.; Zhao, W.; Zhao, P.; Li, M. Adverse physiological and molecular level effects of polystyrene microplastics on freshwater microalgae. Chemosphere 2020, 255, 126914. [Google Scholar] [CrossRef]
- Banaee, M.; Gholamhosseini, A.; Sureda, A.; Soltanian, S.; Fereidouni, M.S.; Ibrahim, A.T.A. Effects of microplastic exposure on the blood biochemical parameters in the pond turtle (Emys orbicularis). Environ. Sci. Pollut. Res. 2021, 28, 9221–9234. [Google Scholar] [CrossRef]
- Abbasi, S.; Soltani, N.; Keshavarzi, B.; Moore, F.; Turner, A.; Hassanaghaei, M. Microplastics in different tissues of fish and prawn from the Musa Estuary, Persian Gulf. Chemosphere 2018, 205, 80–87. [Google Scholar] [CrossRef]
- Daniel, D.B.; Ashraf, P.M.; Thomas, S.N. Microplastics in the edible and inedible tissues of pelagic fishes sold for human consumption in Kerala, India. Environ. Pollut. 2020, 266, 115365. [Google Scholar] [CrossRef]
- Carbery, M.; O’Connor, W.; Palanisami, T. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018, 115, 400–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Gao, H.; Jin, S.; Li, R.; Na, G. The ecotoxicological effects of microplastics on aquatic food web, from primary producer to human: A review. Ecotoxicol. Environ. Saf. 2019, 173, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C. Microplastic in Terrestrial Ecosystems and the Soil? Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, R. Microplastics are not important for the cycling and bioaccumulation of organic pollutants in the oceans—but should microplastics be considered POPs themselves? Integr. Environ. Assess. Manag. 2017, 13, 460–465. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Qiang, L.; Shi, H.; Cheng, J. Bioaccumulation of microplastics and its in vivo interactions with trace metals in edible oysters. Mar. Pollut. Bull. 2020, 154, 111079. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 2016, 351, 1196. [Google Scholar] [CrossRef]
- Campos, D.; Rodrigues, A.C.M.; Rocha, R.J.M.; Martins, R.; Candeias-Mendes, A.; Castanho, S.; Soares, F.; Pousão-Ferreira, P.; Soares, A.M.V.M.; Gravato, C.; et al. Are Microplastics Impairing Marine Fish Larviculture?—Preliminary Results with Argyrosomus regius. Water 2021, 13, 104. [Google Scholar] [CrossRef]
- Cormier, B.; Le Bihanic, F.; Cabar, M.; Crebassa, J.-C.; Blanc, M.; Larsson, M.; Dubocq, F.; Yeung, L.; Clérandeau, C.; Keiter, S.H.; et al. Chronic feeding exposure to virgin and spiked microplastics disrupts essential biological functions in teleost fish. J. Hazard. Mater. 2021, 415, 125626. [Google Scholar] [CrossRef] [PubMed]
- Hansson, T.; Lindesjöö, E.; Förlin, L.; Balk, L.; Bignert, A.; Larsson, Å. Long-term monitoring of the health status of female perch (Perca fluviatilis) in the Baltic Sea shows decreased gonad weight and increased hepatic EROD activity. Aquat. Toxicol. 2006, 79, 341–355. [Google Scholar] [CrossRef]
- Naidoo, T.; Glassom, D. Decreased growth and survival in small juvenile fish, after chronic exposure to environmentally relevant concentrations of microplastic. Mar. Pollut. Bull. 2019, 145, 254–259. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Lu, L.; Zheng, M.; Zhang, X.; Tian, H.; Wang, W.; Ru, S. Polystyrene microplastics cause tissue damages, sex-specific reproductive disruption and transgenerational effects in marine medaka (Oryzias melastigma). Environ. Pollut. 2019, 254, 113024. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Vieira, L.R.; Branco, V.; Figueiredo, N.; Carvalho, F.; Carvalho, C.; Guilhermino, L. Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquat. Toxicol. 2018, 195, 49–57. [Google Scholar] [CrossRef]
- Kim, J.-H.; Yu, Y.-B.; Choi, J.-H. Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and neurotoxicity in fish exposed to microplastics: A review. J. Hazard. Mater. 2021, 413, 125423. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 2020, 717, 134625. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.T.B.; Estrela, F.N.; Pereira, P.S.; de Andrade Vieira, J.E.; de Lima Rodrigues, A.S.; Silva, F.G.; Malafaia, G. Toxicity of polystyrene nanoplastics in Ctenopharyngodon idella juveniles: A genotoxic, mutagenic and cytotoxic perspective. Sci. Total Environ. 2021, 752, 141937. [Google Scholar] [CrossRef]
- Critchell, K.; Hoogenboom, M.O. Effects of microplastic exposure on the body condition and behaviour of planktivorous reef fish (Acanthochromis polyacanthus). PLoS ONE 2018, 13, e0193308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Ge, J.; Yu, X. Bioavailability and toxicity of microplastics to fish species: A review. Ecotoxicol. Environ. Saf. 2020, 189, 109913. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Bobori, D.; Dimitriadi, A.; Karasiali, S.; Tsoumaki-Tsouroufli, P.; Mastora, M.; Kastrinaki, G.; Feidantsis, K.; Printzi, A.; Koumoundouros, G.; Kaloyianni, M. Common mechanisms activated in the tissues of aquatic and terrestrial animal models after TiO2 nanoparticles exposure. Environ. Int. 2020, 138, 105611. [Google Scholar] [CrossRef] [PubMed]
- Kaloyianni, M.; Dimitriadi, A.; Ovezik, M.; Stamkopoulou, D.; Feidantsis, K.; Kastrinaki, G.; Gallios, G.; Tsiaoussis, I.; Koumoundouros, G.; Bobori, D. Magnetite nanoparticles effects on adverse responses of aquatic and terrestrial animal models. J. Hazard. Mater. 2020, 383, 121204. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, C.; Spurgeon, D.J.; Hankard, P.K.; Weeks, J.M. A review of lysosomal membrane stability measured by neutral red retention: Is it a workable earthworm biomarker? Ecotoxicol. Environ. Saf. 2004, 57, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Prokić, M.D.; Radovanović, T.B.; Gavrić, J.P.; Faggio, C. Ecotoxicological effects of microplastics: Examination of biomarkers, current state and future perspectives. TrAC Trends Anal. Chem. 2019, 111, 37–46. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiao, R.; Zhang, S.; Wang, G. Metabolomic profiling reveals the intestinal toxicity of different length of microplastic fibers on zebrafish (Danio rerio). J. Hazard. Mater. 2021, 403, 123663. [Google Scholar] [CrossRef]
- Solomando, A.; Capó, X.; Alomar, C.; Compa, M.; Valencia, J.M.; Sureda, A.; Deudero, S. Assessment of the effect of long-term exposure to microplastics and depuration period in Sparus aurata Linnaeus, 1758: Liver and blood biomarkers. Sci. Total Environ. 2021, 786, 147479. [Google Scholar] [CrossRef]
- Dimitriadi, A.; Papaefthimiou, C.; Genizegkini, E.; Sampsonidis, I.; Kalogiannis, S.; Feidantsis, K.; Bobori, D.C.; Kastrinaki, G.; Koumoundouros, G.; Lambropoulou, D.A.; et al. Adverse effects polystyrene microplastics exert on zebrafish heart—Molecular to individual level. J. Hazard. Mater. 2021, 416, 125969. [Google Scholar] [CrossRef]
- Rodrigues, E.D.L.; Fanta, E. Liver histopathology of the fish Brachydanio rerio Hamilton-Buchman after acute exposure to sublethal levels of the organophosphate Dimethoate 500. Rev. Bras. Zool. 1998, 15, 441–450. [Google Scholar] [CrossRef]
- van der Oost, R.; Beyer, J.; Vermeulen, N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- Handy, R.D.; Al-Bairuty, G.; Al-Jubory, A.; Ramsden, C.S.; Boyle, D.; Shaw, B.J.; Henry, T.B. Effects of manufactured nanomaterials on fishes: A target organ and body systems physiology approach. J. Fish Biol. 2011, 79, 821–853. [Google Scholar] [CrossRef]
- Huuskonen, S.; Lindström-Seppä, P. Hepatic cytochrome P4501A and other biotransformation activities in perch (Perca fluviatilis): The effects of unbleached pulp mill effluents. Aquat. Toxicol. 1995, 31, 27–41. [Google Scholar] [CrossRef]
- Noaksson, E.; Linderoth, M.; Gustavsson, B.; Zebühr, Y.; Balk, L. Reproductive status in female perch (Perca fluviatilis) outside a sewage treatment plant processing leachate from a refuse dump. Sci. Total Environ. 2005, 340, 97–112. [Google Scholar] [CrossRef]
- Laidou, S.; Alanis-Lobato, G.; Pribyl, J.; Raskó, T.; Tichy, B.; Mikulasek, K.; Tsagiopoulou, M.; Oppelt, J.; Kastrinaki, G.; Lefaki, M.; et al. Nuclear inclusions of pathogenic ataxin-1 induce oxidative stress and perturb the protein synthesis machinery. Redox Biol. 2020, 32, 101458. [Google Scholar] [CrossRef]
- Iheanacho, S.C.; Odo, G.E. Dietary exposure to polyvinyl chloride microparticles induced oxidative stress and hepatic damage in Clarias gariepinus (Burchell, 1822). Environ. Sci. Pollut. Res. 2020, 27, 21159–21173. [Google Scholar] [CrossRef] [PubMed]
- Phuong, N.N.; Zalouk-Vergnoux, A.; Poirier, L.; Kamari, A.; Châtel, A.; Mouneyrac, C.; Lagarde, F. Is there any consistency between the microplastics found in the field and those used in laboratory experiments? Environ. Pollut. 2016, 211, 111–123. [Google Scholar] [CrossRef]
- Nerantzaki, M.; Adam, K.-V.; Koliakou, I.; Skoufa, E.; Avgeropoulos, A.; Papageorgiou, G.Z.; Bikiaris, D. Novel Castor Oil-Derived Block Copolymers as Promising Candidates for Biological Applications: Biorelevant and Biocompatible. Macromol. Chem. Phys. 2017, 218, 1700305. [Google Scholar] [CrossRef]
- Lowe, D.M.; Pipe, R.K. Contaminant induced lysosomal membrane damage in marine mussel digestive cells: An in vitro study. Aquat. Toxicol. 1994, 30, 357–365. [Google Scholar] [CrossRef]
- Dailianis, S.; Domouhtsidou, G.P.; Raftopoulou, E.; Kaloyianni, M.; Dimitriadis, V.K. Evaluation of neutral red retention assay, micronucleus test, acetylcholinesterase activity and a signal transduction molecule (cAMP) in tissues of Mytilus galloprovincialis (L.), in pollution monitoring. Mar. Environ. Res. 2003, 56, 443–470. [Google Scholar] [CrossRef]
- Rainieri, S.; Conlledo, N.; Larsen, B.K.; Granby, K.; Barranco, A. Combined effects of microplastics and chemical contaminants on the organ toxicity of zebrafish (Danio rerio). Environ. Res. 2018, 162, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Solomando, A.; Capó, X.; Alomar, C.; Álvarez, E.; Compa, M.; Valencia, J.M.; Pinya, S.; Deudero, S.; Sureda, A. Long-term exposure to microplastics induces oxidative stress and a pro-inflammatory response in the gut of Sparus aurata Linnaeus, 1758. Environ. Pollut. 2020, 266, 115295. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, W.G., Jr.; Samuelsson, B. Formation of Malonaldehyde from Phospholipid Arachidonate during Microsomal Lipid Peroxidation. Eur. J. Biochem. 1968, 6, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Wills, E.D. Lipid peroxide formation in microsomes. General considerations. Biochem. J. 1969, 113, 315–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buss, H.; Chan, T.P.; Sluis, K.B.; Domigan, N.M.; Winterbourn, C.C. Protein Carbonyl Measurement by a Sensitive ELISA Method. Free Radic. Biol. Med. 1997, 23, 361–366. [Google Scholar] [CrossRef]
- Alamdari, D.H.; Kostidou, E.; Paletas, K.; Sarigianni, M.; Konstas, A.G.P.; Karapiperidou, A.; Koliakos, G. High sensitivity enzyme-linked immunosorbent assay (ELISA) method for measuring protein carbonyl in samples with low amounts of protein. Free Radic. Biol. Med. 2005, 39, 1362–1367. [Google Scholar] [CrossRef]
- Dailianis, S.; Piperakis, S.M.; Kaloyianni, M. Cadmium effects on ROS production and DNA damage via adrenergic receptors stimulation: Role of Na+/H+ exchanger and PKC. Free Radic. Res. 2005, 39, 1059–1070. [Google Scholar] [CrossRef]
- Liao, W.; McNutt, M.A.; Zhu, W.-G. The comet assay: A sensitive method for detecting DNA damage in individual cells. Methods 2009, 48, 46–53. [Google Scholar] [CrossRef]
- Ritter, D.; Knebel, J. Genotoxicity testing in vitro—Development of a higher throughput analysis method based on the comet assay. Toxicol. In Vitro 2009, 23, 1570–1575. [Google Scholar] [CrossRef]
- Binelli, A.; Cogni, D.; Parolini, M.; Riva, C.; Provini, A. In vivo experiments for the evaluation of genotoxic and cytotoxic effects of Triclosan in Zebra mussel hemocytes. Aquat. Toxicol. 2009, 91, 238–244. [Google Scholar] [CrossRef]
- Virgiliou, C.; Sampsonidis, I.; Gika, H.G.; Raikos, N.; Theodoridis, G.A. Development and validation of a HILIC-MS/MS multitargeted method for metabolomics applications. Electrophoresis 2015, 36, 2215–2225. [Google Scholar] [CrossRef] [PubMed]
- Zisi, C.; Sampsonidis, I.; Fasoula, S.; Papachristos, K.; Witting, M.; Gika, H.G.; Nikitas, P.; Pappa-Louisi, A. QSRR Modeling for Metabolite Standards Analyzed by Two Different Chromatographic Columns Using Multiple Linear Regression. Metabolites 2017, 7, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, F.S. Applications of Infrared Spectroscopy in Biochemistry, Biology, and Medicine, 1st ed.; Springer: Boston, MA, USA, 1971. [Google Scholar]
- Kastrinaki, G.; Samsouris, C.; Kosmidis, E.K.; Papaioannou, E.; Konstandopoulos, A.G.; Theophilidis, G. Assessing the axonal translocation of CeO2 and SiO2 nanoparticles in the sciatic nerve fibers of the frog: An ex vivo electrophysiological study. Int. J. Nanomed. 2015, 10, 7089–7096. [Google Scholar] [CrossRef] [Green Version]
- Sheng, C.; Zhang, S.; Zhang, Y. The influence of different polymer types of microplastics on adsorption, accumulation, and toxicity of triclosan in zebrafish. J. Hazard. Mater. 2021, 402, 123733. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Ma, Z.; Li, F.; Duan, C.; Yuan, Y.; Zhu, C.; Wang, L.; Zhu, X.; Wang, S.; Gao, P.; et al. Hypoxanthine Induces Muscular ATP Depletion and Fatigue via UCP2. Front. Physiol. 2021, 12, 647743. [Google Scholar] [CrossRef]
- Xia, X.; Sun, M.; Zhou, M.; Chang, Z.; Li, L. Polyvinyl chloride microplastics induce growth inhibition and oxidative stress in Cyprinus carpio var. larvae. Sci. Total Environ. 2020, 716, 136479. [Google Scholar] [CrossRef]
- Indo, H.P.; Davidson, M.; Yen, H.-C.; Suenaga, S.; Tomita, K.; Nishii, T.; Higuchi, M.; Koga, Y.; Ozawa, T.; Majima, H.J. Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage. Mitochondrion 2007, 7, 106–118. [Google Scholar] [CrossRef]
- Taze, C.; Panetas, I.; Kalogiannis, S.; Feidantsis, K.; Gallios, G.P.; Kastrinaki, G.; Konstandopoulos, A.G.; Václavíková, M.; Ivanicova, L.; Kaloyianni, M. Toxicity assessment and comparison between two types of iron oxide nanoparticles in Mytilus galloprovincialis. Aquat. Toxicol. 2016, 172, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.-G.; Ahn, B.-W.; Shaltiel, S.; Stadtman, E.R. [49] Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef]
- Ghezzi, P.; Bonetto, V. Redox proteomics: Identification of oxidatively modified proteins. Proteomics 2003, 3, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Hecker, M.; Wagner, A.H. Role of protein carbonylation in diabetes. J. Inher. Metab. Dis. 2018, 41, 29–38. [Google Scholar] [CrossRef]
- Kaloyianni, M.; Feidantsis, K.; Nteli, I.; Stergiou, P.; Tsoulia, T.; Dimitriadi, A.; Antonopoulou, E.; Bobori, D. Biochemical and molecular responses of cyprinids in two Mediterranean lacustrine ecosystems: Opportunities for ecological assessment and biomonitoring. Aquat. Toxicol. 2019, 211, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Jin, S.-R.; Chen, Z.-Z.; Gao, J.-Z.; Liu, Y.-N.; Liu, J.-H.; Feng, X.-S. Single and combined effects of microplastics and cadmium on the cadmium accumulation, antioxidant defence and innate immunity of the discus fish (Symphysodon aequifasciatus). Environ. Pollut. 2018, 243, 462–471. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pannetier, P.; Morin, B.; Le Bihanic, F.; Dubreil, L.; Clérandeau, C.; Chouvellon, F.; Van Arkel, K.; Danion, M.; Cachot, J. Environmental samples of microplastics induce significant toxic effects in fish larvae. Environ. Int. 2020, 134, 105047. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; d’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef]
- Ribeiro, F.; Garcia, A.R.; Pereira, B.P.; Fonseca, M.; Mestre, N.C.; Fonseca, T.G.; Ilharco, L.M.; Bebianno, M.J. Microplastics effects in Scrobicularia plana. Mar. Pollut. Bull. 2017, 122, 379–391. [Google Scholar] [CrossRef]
- Yang, H.; Lai, H.; Huang, J.; Sun, L.; Mennigen, J.A.; Wang, Q.; Liu, Y.; Jin, Y.; Tu, W. Polystyrene microplastics decrease F–53B bioaccumulation but induce inflammatory stress in larval zebrafish. Chemosphere 2020, 255, 127040. [Google Scholar] [CrossRef]
- Capó, X.; Company, J.J.; Alomar, C.; Compa, M.; Sureda, A.; Grau, A.; Hansjosten, B.; López-Vázquez, J.; Quintana, J.B.; Rodil, R.; et al. Long-term exposure to virgin and seawater exposed microplastic enriched-diet causes liver oxidative stress and inflammation in gilthead seabream Sparus aurata, Linnaeus 1758. Sci. Total Environ. 2021, 767, 144976. [Google Scholar] [CrossRef]
- Alomar, C.; Sureda, A.; Capó, X.; Guijarro, B.; Tejada, S.; Deudero, S. Microplastic ingestion by Mullus surmuletus Linnaeus, 1758 fish and its potential for causing oxidative stress. Environ. Res. 2017, 159, 135–142. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, S.; Razanajatovo, R.M.; Zou, H.; Zhu, W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Magni, S.; Della Torre, C.; Garrone, G.; D’Amato, A.; Parenti, C.C.; Binelli, A. First evidence of protein modulation by polystyrene microplastics in a freshwater biological model. Environ. Pollut. 2019, 250, 407–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, F.; Taylor, A. Oxidative stress and recovery from oxidative stress are associated with altered ubiquitin conjugating and proteolytic activities in bovine lens epithelial cells. Biochem. J. 1995, 307 Pt 1, 297–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chondrogianni, N.; Petropoulos, I.; Grimm, S.; Georgila, K.; Catalgol, B.; Friguet, B.; Grune, T.; Gonos, E.S. Protein damage, repair and proteolysis. Mol. Asp. Med. 2014, 35, 1–71. [Google Scholar] [CrossRef]
- Jing, K.; Shin, S.; Jeong, S.; Kim, S.; Song, K.S.; Park, J.H.; Heo, J.Y.; Seo, K.S.; Park, S.K.; Kweon, G.R.; et al. Docosahexaenoic acid induces the degradation of HPV E6/E7 oncoproteins by activating the ubiquitin–proteasome system. Cell Death Dis. 2014, 5, e1524. [Google Scholar] [CrossRef] [Green Version]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.-H.; Yang, F.-F.; Liao, S.-A.; Miao, Y.-T.; Ye, C.-X.; Wang, A.-L.; Tan, J.-W.; Chen, X.-Y. High temperature induces apoptosis and oxidative stress in pufferfish (Takifugu obscurus) blood cells. J. Therm. Biol. 2015, 53, 172–179. [Google Scholar] [CrossRef]
- Jiang, W.-D.; Feng, L.; Qu, B.; Wu, P.; Kuang, S.-Y.; Jiang, J.; Tang, L.; Tang, W.-N.; Zhang, Y.-A.; Zhou, X.-Q.; et al. Changes in integrity of the gill during histidine deficiency or excess due to depression of cellular anti-oxidative ability, induction of apoptosis, inflammation and impair of cell-cell tight junctions related to Nrf2, TOR and NF-κB signaling in fish. Fish Shellfish Immunol. 2016, 56, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Takle, H.; Andersen, Ø. Caspases and apoptosis in fish. J. Fish Biol. 2007, 71, 326–349. [Google Scholar] [CrossRef]
- Wang, K. Autophagy and apoptosis in liver injury. Cell Cycle 2015, 14, 1631–1642. [Google Scholar] [CrossRef] [Green Version]
- Fairlie, W.D.; Tran, S.; Lee, E.F. Chapter Four—Crosstalk between apoptosis and autophagy signaling pathways. In International Review of Cell and Molecular Biology; Spetz, J.K.E., Galluzzi, L., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 352, pp. 115–158. [Google Scholar]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef] [Green Version]
- Kabeya, Y.; Mizushima, N.; Yamamoto, A.; Oshitani-Okamoto, S.; Ohsumi, Y.; Yoshimori, T. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J. Cell Sci. 2004, 117, 2805–2812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjørkøy, G.; Lamark, T.; Johansen, T. p62/SQSTM1: A Missing Link between Protein Aggregates and the Autophagy Machinery. Autophagy 2006, 2, 138–139. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, M.; Waguri, S.; Koike, M.; Sou, Y.-S.; Ueno, T.; Hara, T.; Mizushima, N.; Iwata, J.-I.; Ezaki, J.; Murata, S.; et al. Homeostatic Levels of p62 Control Cytoplasmic Inclusion Body Formation in Autophagy-Deficient Mice. Cell 2007, 131, 1149–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.-A.; Outzen, H.; Øvervatn, A.; Bjørkøy, G.; Johansen, T. p62/SQSTM1 Binds Directly to Atg8/LC3 to Facilitate Degradation of Ubiquitinated Protein Aggregates by Autophagy. J. Biol. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef] [Green Version]
- Dodson, M.; Darley-Usmar, V.; Zhang, J. Cellular metabolic and autophagic pathways: Traffic control by redox signaling. Free Radic. Biol. Med. 2013, 63, 207–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.S.; Jung, Y.-J.; Hong, N.-H.; Hong, S.H.; Park, J.-W. Toxicological effects of irregularly shaped and spherical microplastics in a marine teleost, the sheepshead minnow (Cyprinodon variegatus). Mar. Pollut. Bull. 2018, 129, 231–240. [Google Scholar] [CrossRef]
- Limonta, G.; Mancia, A.; Benkhalqui, A.; Bertolucci, C.; Abelli, L.; Fossi, M.C.; Panti, C. Microplastics induce transcriptional changes, immune response and behavioral alterations in adult zebrafish. Sci. Rep. 2019, 9, 15775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Moos, N.; Burkhardt-Holm, P.; Köhler, A. Uptake and Effects of Microplastics on Cells and Tissue of the Blue Mussel Mytilus edulis L. after an Experimental Exposure. Environ. Sci. Technol. 2012, 46, 11327–11335. [Google Scholar] [CrossRef]
- Gambino, G.; Falleni, A.; Nigro, M.; Salvetti, A.; Cecchettini, A.; Ippolito, C.; Guidi, P.; Rossi, L. Dynamics of interaction and effects of microplastics on planarian tissue regeneration and cellular homeostasis. Aquat. Toxicol. 2020, 218, 105354. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhang, Y.; Lu, Y.; He, L.; Qu, J.; Zhou, C.; Hong, P.; Sun, S.; Zhao, H.; Liang, Y.; et al. The Complex Toxicity of Tetracycline with Polystyrene Spheres on Gastric Cancer Cells. Int. J. Environ. Res. Public Health 2020, 17, 2808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawlor, C.; O’Connor, G.; O’Leary, S.; Gallagher, P.J.; Cryan, S.-A.; Keane, J.; O’Sullivan, M.P. Treatment of Mycobacterium tuberculosis-Infected Macrophages with Poly(Lactic-Co-Glycolic Acid) Microparticles Drives NFκB and Autophagy Dependent Bacillary Killing. PLoS ONE 2016, 11, e0149167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Teng, J.; Zhao, J.; Zhu, X.; Shan, E.; Zhang, C.; Zhang, W.; Wang, Q. Toxic effects of exposure to microplastics with environmentally relevant shapes and concentrations: Accumulation, energy metabolism and tissue damage in oyster Crassostrea gigas. Environ. Pollut. 2021, 269, 116169. [Google Scholar] [CrossRef]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, C.; Zhou, J.; Shen, M.; Wang, X.; Fu, Z.; Jin, Y. Effects of polystyrene microplastics on the composition of the microbiome and metabolism in larval zebrafish. Chemosphere 2019, 217, 646–658. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, Z.; Huang, Z.; Bao, Z.; Luo, T.; Jin, Y. Effects of polyethylene microplastics on the microbiome and metabolism in larval zebrafish. Environ. Pollut. 2021, 282, 117039. [Google Scholar] [CrossRef]
- Zhao, H.-J.; Xu, J.-K.; Yan, Z.-H.; Ren, H.-Q.; Zhang, Y. Microplastics enhance the developmental toxicity of synthetic phenolic antioxidants by disturbing the thyroid function and metabolism in developing zebrafish. Environ. Int. 2020, 140, 105750. [Google Scholar] [CrossRef]
- Currie, S.; Moyes, C.D.; Tufts, B.L. The effects of heat shock and acclimation temperature on hsp70 and hsp30 mRNA expression in rainbow trout: In vivo and in vitro comparisons. J. Fish Biol. 2000, 56, 398–408. [Google Scholar] [CrossRef]
- Dietz, T.J.; Somero, G.N. The threshold induction temperature of the 90-kDa heat shock protein is subject to acclimatization in eurythermal goby fishes (genus Gillichthys). Proc. Natl. Acad. Sci. USA 1992, 89, 3389. [Google Scholar] [CrossRef] [Green Version]
- Vignardi, C.P.; Hasue, F.M.; Sartório, P.V.; Cardoso, C.M.; Machado, A.S.D.; Passos, M.J.A.C.R.; Santos, T.C.A.; Nucci, J.M.; Hewer, T.L.R.; Watanabe, I.-S.; et al. Genotoxicity, potential cytotoxicity and cell uptake of titanium dioxide nanoparticles in the marine fish Trachinotus carolinus (Linnaeus, 1766). Aquat. Toxicol. 2015, 158, 218–229. [Google Scholar] [CrossRef]
- Greven, A.-C.; Merk, T.; Karagöz, F.; Mohr, K.; Klapper, M.; Jovanović, B.; Palić, D. Polycarbonate and polystyrene nanoplastic particles act as stressors to the innate immune system of fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 2016, 35, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Greven, A.-C. Polycarbonate and Polystyrene Nanoparticles Act as Stressors to the Innate Immune System of Fathead Minnows (Pimephales promelas, Rafinesque 1820). Ph.D. Thesis, LMU München-Tierärztliche Fakultät, Munich, Germany, 2016. [Google Scholar]
- Kashiwada, S. Distribution of Nanoparticles in the See-through Medaka (Oryzias latipes). Environ. Health Perspect. 2006, 114, 1697–1702. [Google Scholar] [CrossRef] [Green Version]
- Yong, C.Q.Y.; Valiyaveetill, S.; Tang, B.L. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef] [Green Version]
- Barrera, G. Oxidative Stress and Lipid Peroxidation Products in Cancer Progression and Therapy. ISRN Oncol. 2012, 2012, 137289. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Parameter | Species | Tissue | Species -Tissue |
|---|---|---|---|
| MDA | ns | ns | F(1,24) = 7.14, p = 0.013 |
| Car | ns | ns | F(1,24) = 36.8, p = 0.001 |
| Ub | ns | ns | F(1,24) = 7.74, p = 0.010 |
| DNA | ns | ns | F(1,24) = 7.67, p = 0.011 |
| Bax/Bcl-2 | ns | ns | ns |
| Cas | ns | ns | F(1,24) = 57.86, p = 0.000 |
| LC3 II/I | ns | ns | F(1,24) = 144.50, p = 0.000 |
| Danio rerio | Perca fluviatilis | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA | MDA | Car | Ub | LC3 II/I | Bax/Bcl-2 | SQSTM1/p62 | Cas | DNA | MDA | Car | Ub | LC3 II/I | Bax/Bcl-2 | SQSTM1/p62 | Cas | |
| DNA | − | |||||||||||||||
| MDA | + | + | + | |||||||||||||
| Car | + | + | − | |||||||||||||
| Ub | + | |||||||||||||||
| LC3 II/I | + | + | + | |||||||||||||
| Bax/Bcl-3 | + | + | + | − | ||||||||||||
| SQSTM1/p62 | − | − | − | − | ||||||||||||
| Cas | + | − | − | − | − | + | ||||||||||
| liver | gills | (+) possitive | (−) negative | p < 0.05 | ||||||||||||
| Gills | Liver | |||
|---|---|---|---|---|
| Zebrafish | Perch | Zebrafish | Perch | |
| L-Asparagine | −78 | 99 | 61 | |
| L-Glutamine | −36 | −15 | ||
| L-Glutamic acid | −83 | −86 | −8 | −21 |
| L-Valine | −79 | −63 | 194 | 21 |
| L-Lysine | −72 | −23 | −14 | |
| L-Alanine | −83 | −86 | 55 | −30 |
| L-Proline | −91 | −63 | 96 | 8 |
| L-Tyrosine | −84 | 3 | 67 | 45 |
| L-Phenylalanine | −93 | 64 | 113 | 67 |
| L-Arginine | −51 | 135 | 221 | |
| Ornithine | −15 | −5 | ||
| Citrulline | −67 | 88 | −58 | |
| Creatine | 59 | −34 | ||
| Creatinine | 62 | −57 | ||
| Pyruvic acid | −75 | |||
| L-Lactic acid | −44 | −66 | ||
| Succinic acid | −56 | −54 | 20 | 120 |
| 2-Oxoglutaric acid | −31 | −61 | ||
| L-Carnitine | −91 | −61 | 96 | 50 |
| ALCAR | −80 | −96 | −55 | −56 |
| Butyric acid | 28 | 28 | ||
| Hypoxanthine | 223 | −59 | ||
| Adenine | −56 | −54 | ||
| Adenosine | −65 | 120 | ||
| Deoxyadenosine | 124 | −44 | ||
| Uridine | 155 | −45 | ||
| Salicylic acid | −30 | 210 | −51 | 7 |
| Betaine | −70 | −45 | −11 | 75 |
| Choline | −49 | −33 | 94 | 8 |
| Putrescine | −43 | |||
| Niacinamide | −71 | −59 | 7 | 17 |
| Riboflavin | 1 | |||
| Trehalose | −74 | −81 | ||
| Metabolites | Species | Tissue | Species -Tissue |
|---|---|---|---|
| Betaine | ns | ns | F(1,8) = 124.09, p = 0.000 |
| Choline | ns | ns | F(1,8) = 270.74, p = 0.000 |
| L-alanine | ns | ns | F(1,8) = 23.83, p = 0.001 |
| L-carnitine | ns | ns | F(1,8) = 154.40, p = 0.000 |
| L-glutamic acid | F(1,1) = 6384.34, p = 0.008 | F(1,1) = 531,344, p = 0.001 | ns |
| L-phenylalanine | ns | ns | F(1,8) = 139.07, p = 0.000 |
| L-proline | ns | ns | F(1,8) = 14.61, p = 0.005 |
| Salicylic acid | ns | ns | F(1,8) = 380.88, p = 0.000 |
| Succinic acid | ns | ns | F(1,8) = 4.75, p = 0.061 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaloyianni, M.; Bobori, D.C.; Xanthopoulou, D.; Malioufa, G.; Sampsonidis, I.; Kalogiannis, S.; Feidantsis, K.; Kastrinaki, G.; Dimitriadi, A.; Koumoundouros, G.; et al. Toxicity and Functional Tissue Responses of Two Freshwater Fish after Exposure to Polystyrene Microplastics. Toxics 2021, 9, 289. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics9110289
Kaloyianni M, Bobori DC, Xanthopoulou D, Malioufa G, Sampsonidis I, Kalogiannis S, Feidantsis K, Kastrinaki G, Dimitriadi A, Koumoundouros G, et al. Toxicity and Functional Tissue Responses of Two Freshwater Fish after Exposure to Polystyrene Microplastics. Toxics. 2021; 9(11):289. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics9110289
Chicago/Turabian StyleKaloyianni, Martha, Dimitra C. Bobori, Despoina Xanthopoulou, Glykeria Malioufa, Ioannis Sampsonidis, Stavros Kalogiannis, Konstantinos Feidantsis, Georgia Kastrinaki, Anastasia Dimitriadi, George Koumoundouros, and et al. 2021. "Toxicity and Functional Tissue Responses of Two Freshwater Fish after Exposure to Polystyrene Microplastics" Toxics 9, no. 11: 289. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics9110289